Trichinellosis is an illness caused by infection with Trichinella. The most common causative parasite is Trichinella spiralis, which is usually found in pigs and transmitted to humans through the ingestion of undercooked meat. Once ingested, the parasite grows and matures within the intestinal walls. The adult forms mate, and the larvae Larvae Wormlike or grublike stage, following the egg in the life cycle of insects, worms, and other metamorphosing animals. Ascaris/Ascariasis produced spread through the bloodstream, reaching striated muscles. Symptoms occur during larval migration. Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship may have GI symptoms within a few weeks after consumption of the infected meat, and systemic symptoms such as fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever, chills Chills The sudden sensation of being cold. It may be accompanied by shivering. Fever, myalgia Myalgia Painful sensation in the muscles. Ion Channel Myopathy, and periorbital Periorbital Orbital and Preseptal Cellulitis edema Edema Edema is a condition in which excess serous fluid accumulates in the body cavity or interstitial space of connective tissues. Edema is a symptom observed in several medical conditions. It can be categorized into 2 types, namely, peripheral (in the extremities) and internal (in an organ or body cavity). Edema may follow. Diagnosis can be made by serologic examination and confirmed by the presence of cysts Cysts Any fluid-filled closed cavity or sac that is lined by an epithelium. Cysts can be of normal, abnormal, non-neoplastic, or neoplastic tissues. Fibrocystic Change or larvae Larvae Wormlike or grublike stage, following the egg in the life cycle of insects, worms, and other metamorphosing animals. Ascaris/Ascariasis in a muscle biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma. Mild infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease are self-limited, but systemic disease is managed with antiparasitic medications and corticosteroids Corticosteroids Chorioretinitis. Infection can be prevented by proper meat handling and cooking techniques.
Last updated: Jan 24, 2025
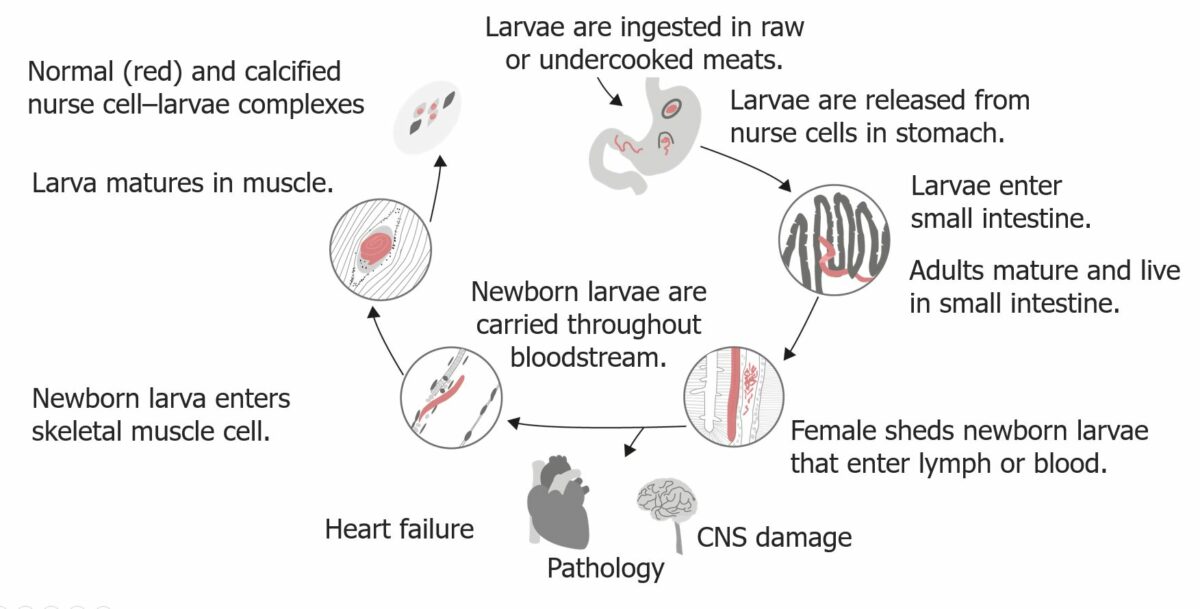
The life cycle of Trichinella spiralis in humans:
There are two phases in humans: the intestinal phase and the systemic phase (larvae get into the circulation and can reach myocardium and the brain, leading to cardiac and cerebral inflammatory reactions).
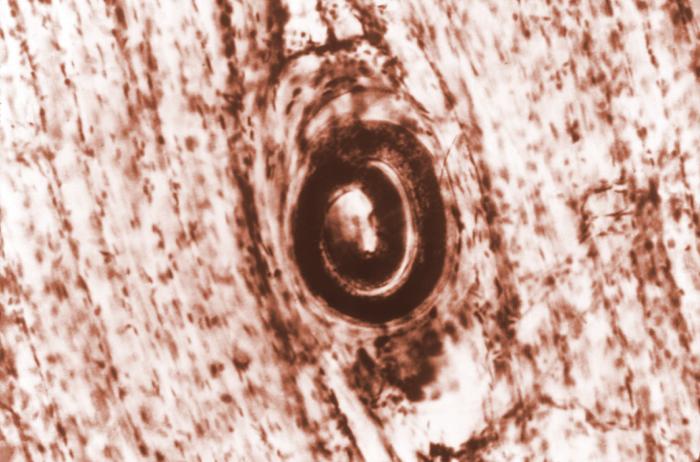
Trichinella spiralis cyst embedded in a muscle tissue specimen in a case of trichinellosis:
Trichinellos is acquired by ingesting meat containing cysts (encysted larvae) of the roundworm parasite.