Lymphocytic choriomeningitis virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology (LCMV) is a single-stranded RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology transmitted to humans via rodents, the primary reservoir Reservoir Animate or inanimate sources which normally harbor disease-causing organisms and thus serve as potential sources of disease outbreaks. Reservoirs are distinguished from vectors (disease vectors) and carriers, which are agents of disease transmission rather than continuing sources of potential disease outbreaks. Humans may serve both as disease reservoirs and carriers. Escherichia coli. Viral infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease can occur through direct contact (such as through a break in the skin Skin The skin, also referred to as the integumentary system, is the largest organ of the body. The skin is primarily composed of the epidermis (outer layer) and dermis (deep layer). The epidermis is primarily composed of keratinocytes that undergo rapid turnover, while the dermis contains dense layers of connective tissue. Skin: Structure and Functions) with rodent urine, saliva Saliva The clear, viscous fluid secreted by the salivary glands and mucous glands of the mouth. It contains mucins, water, organic salts, and ptyalin. Salivary Glands: Anatomy, or droppings or via inhalation of aerosolized virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology. Vertical transmission Vertical transmission The transmission of infectious disease or pathogens from one generation to another. It includes transmission in utero or intrapartum by exposure to blood and secretions, and postpartum exposure via breastfeeding. Congenital TORCH Infections and, rarely, organ transplantation Organ Transplantation Transplantation is a procedure that involves the removal of an organ or living tissue and placing it into a different part of the body or into a different person. Organ transplantations have become the therapeutic option of choice for many individuals with end-stage organ failure. Organ Transplantation also lead to infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease. The disease typically results in a self-limited, febrile, biphasic disease. In severe cases, patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship can present with encephalitis Encephalitis Encephalitis is inflammation of the brain parenchyma caused by an infection, usually viral. Encephalitis may present with mild symptoms such as headache, fever, fatigue, and muscle and joint pain or with severe symptoms such as seizures, altered consciousness, and paralysis. Encephalitis, aseptic meningitis Meningitis Meningitis is inflammation of the meninges, the protective membranes of the brain, and spinal cord. The causes of meningitis are varied, with the most common being bacterial or viral infection. The classic presentation of meningitis is a triad of fever, altered mental status, and nuchal rigidity. Meningitis, or meningoencephalitis Meningoencephalitis Encephalitis. Lymphocytic choriomeningitis virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease of pregnant women have also been recognized as a significant teratogen resulting in fetal death or congenital Congenital Chorioretinitis defects. Diagnosis is by serology Serology The study of serum, especially of antigen-antibody reactions in vitro. Yellow Fever Virus or RT-PCR RT-PCR A variation of the pcr technique in which cDNA is made from RNA via reverse transcription. The resultant cDNA is then amplified using standard pcr protocols. Polymerase Chain Reaction (PCR). Management is supportive.
Last updated: Nov 7, 2022
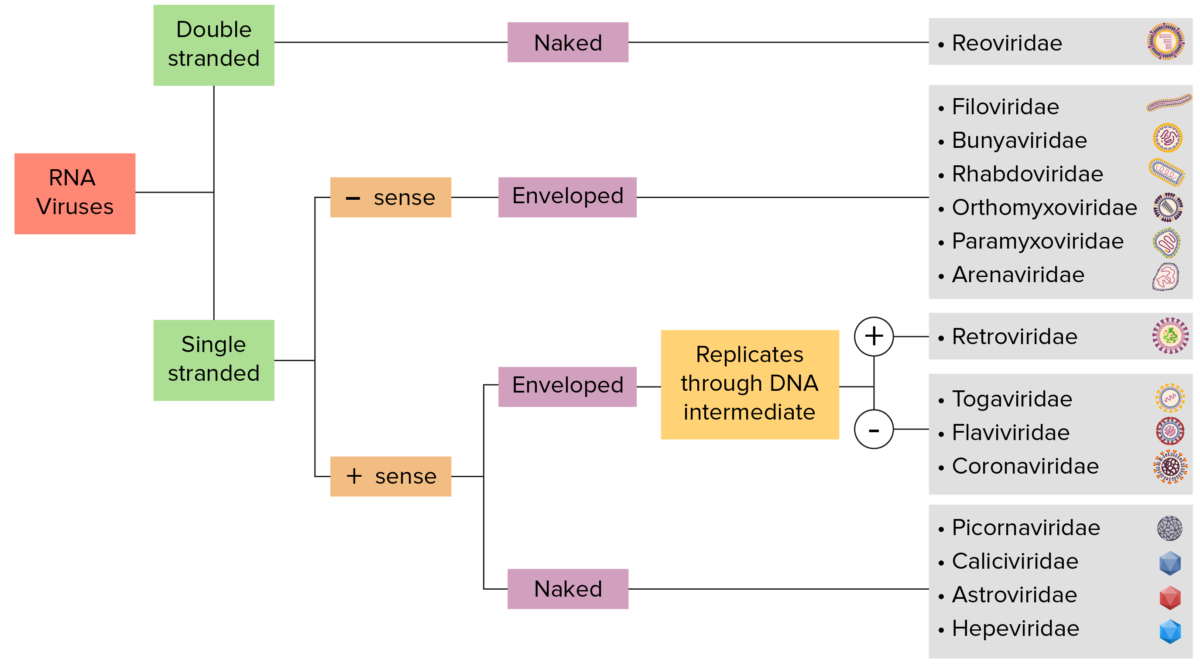
RNA virus identification:
Viruses can be classified in many ways. Most viruses, however, will have a genome formed by either DNA or RNA. RNA genome viruses can be further characterized by either a single- or double-stranded RNA. “Enveloped” viruses are covered by a thin coat of cell membrane (usually taken from the host cell). If the coat is absent, the viruses are called “naked” viruses. Viruses with single-stranded genomes are “positive-sense” viruses if the genome is directly employed as messenger RNA (mRNA), which is translated into proteins. “Negative-sense,” single-stranded viruses employ RNA dependent RNA polymerase, a viral enzyme, to transcribe their genome into messenger RNA.
Taxonomy:
Electron micrograph of lymphocytic choriomeningitis virus
Image: “Negative stain electron micrograph of an arenavirus from a mouse that tested positive for LCM” by CDC. License: Public DomainArenaviruses: cause chronic infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease in rodents (house mouse, Mus musculus)
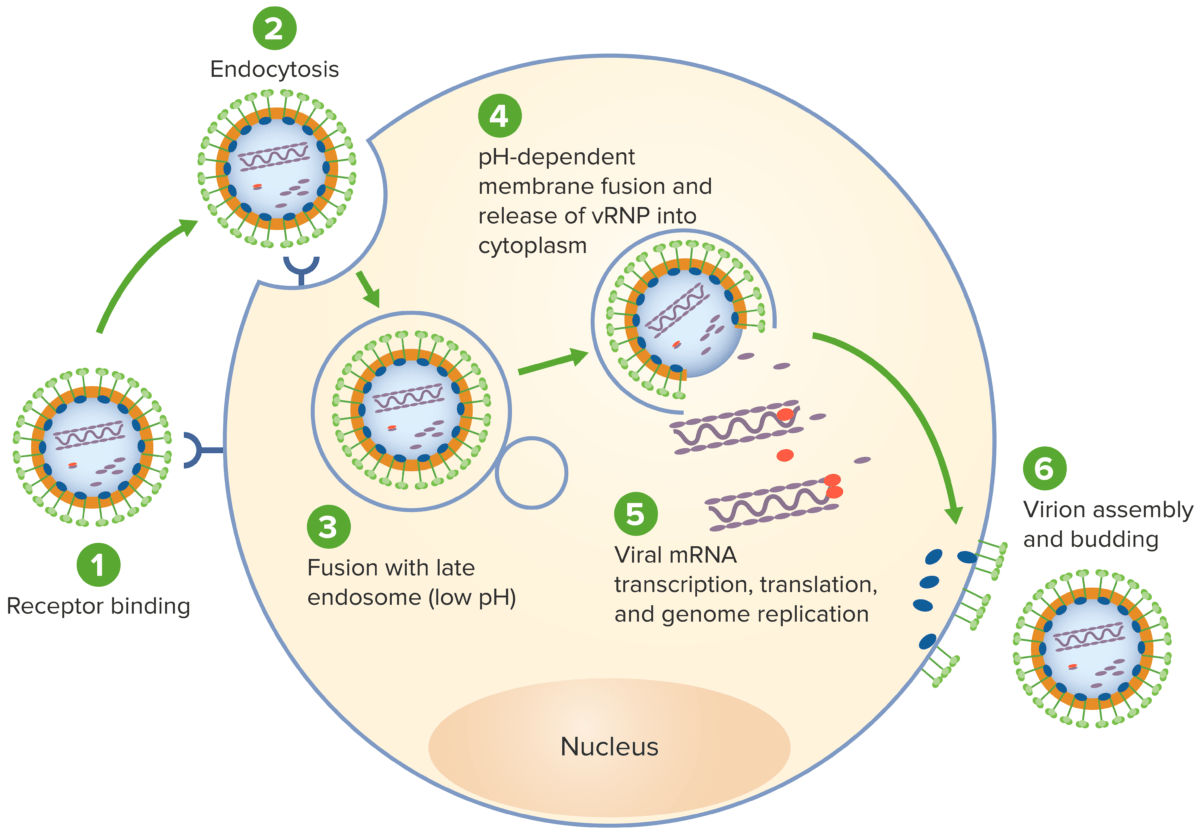
The life cycle of lymphocytic choriomeningitis virus:
1. Virus attaches to host receptors through envelope glycoproteins.
2. Virus is subsequently endocytosed, thus gaining cell entry.
3. Viral fusion with an endosome follows.
4. The virus fuses with the endosome membrane, allowing release of the viral ribonucleocapsid into the cytoplasm.
5. The RNA-dependent RNA polymerase (RdRP) mediates the viral gene replication and transcription in the host cytoplasm.
6. New virions are assembled and budding occurs, releasing the virion to infect other cells.
vRNP: viral ribonucleoprotein
LCMV is not cytotoxic Cytotoxic Parvovirus B19, but while the host immune response tries to eliminate the virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology, this may also trigger Trigger The type of signal that initiates the inspiratory phase by the ventilator Invasive Mechanical Ventilation immune-mediated disease. The immune response produces the different manifestations of the disease.
Infection with LCMV results in a biphasic illness.
Symptom onset:
1st-phase symptoms:
2nd-phase symptoms:
Following a few days of recovery, 2nd-phase symptoms may start, which include neurologic symptoms (typical of meningitis Meningitis Meningitis is inflammation of the meninges, the protective membranes of the brain, and spinal cord. The causes of meningitis are varied, with the most common being bacterial or viral infection. The classic presentation of meningitis is a triad of fever, altered mental status, and nuchal rigidity. Meningitis and/or encephalitis Encephalitis Encephalitis is inflammation of the brain parenchyma caused by an infection, usually viral. Encephalitis may present with mild symptoms such as headache, fever, fatigue, and muscle and joint pain or with severe symptoms such as seizures, altered consciousness, and paralysis. Encephalitis).
Other less frequent findings:
Women who become infected with LCMV during pregnancy Pregnancy The status during which female mammals carry their developing young (embryos or fetuses) in utero before birth, beginning from fertilization to birth. Pregnancy: Diagnosis, Physiology, and Care can pass the infection to the fetus.
Significant immunosuppression in transplant recipients play a role in the development of disease:
During the 1st phase of the disease, the most common laboratory abnormalities are
Arenaviruses can cause neurologic disease (LCMV) and/or hemorrhagic fevers, for which one of the major etiologies is the Lassa virus Lassa Virus Lassa virus, part of the Arenaviridae family, is an ssRNA virus that causes Lassa fever, a type of viral hemorrhagic illness. The virus is endemic in parts of West Africa (Sierra Leone, Liberia, Guinea, and Nigeria) and neighboring countries. The reservoir is the multimammate rat (Mastomys natalensis), and transmission is via inhalation or contact with rodent excretions or consumption of rodents. Lassa Virus.
| Organism | LCMV | Lassa virus Lassa Virus Lassa virus, part of the Arenaviridae family, is an ssRNA virus that causes Lassa fever, a type of viral hemorrhagic illness. The virus is endemic in parts of West Africa (Sierra Leone, Liberia, Guinea, and Nigeria) and neighboring countries. The reservoir is the multimammate rat (Mastomys natalensis), and transmission is via inhalation or contact with rodent excretions or consumption of rodents. Lassa Virus |
|---|---|---|
| Family | Arenaviridae Arenaviridae A family of RNA viruses naturally infecting rodents and consisting of one genus (arenavirus) with two groups: old world arenaviruses and new world arenaviruses. Infection in rodents is persistent and silent. Vertical transmission is through milk-, saliva-, or urine-borne routes. Horizontal transmission to humans, monkeys, and other animals is important. Lassa Virus | |
| Genus | Mammarenavirus Mammarenavirus Lassa Virus | |
| Characteristics |
|
|
| Reservoir Reservoir Animate or inanimate sources which normally harbor disease-causing organisms and thus serve as potential sources of disease outbreaks. Reservoirs are distinguished from vectors (disease vectors) and carriers, which are agents of disease transmission rather than continuing sources of potential disease outbreaks. Humans may serve both as disease reservoirs and carriers. Escherichia coli | Rodents | |
| Transmission |
|
|
| Clinical course | Biphasic:
|
|
| Diagnosis |
|
|
| Management | Supportive |
|
| Prevention | Avoid contact with rodents and their body fluids. |
|
The following selected RNA viruses RNA Viruses Viruses whose genetic material is RNA. Virology can also lead to CNS infection.