Bunyaviridae is a family of RNA viruses RNA Viruses Viruses whose genetic material is RNA. Virology that is classified into 5 genera: Orthobunyavirus (La Crosse virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology), Hantavirus, Nairovirus (Crimean-Congo hemorrhagic fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology), Phlebovirus (Rift Valley fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology), and Tospovirus. The common characteristics of the virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology family include an enveloped spherical structure containing a single-stranded, negative-sense RNA Negative-sense RNA RNA viruses that have their genetic material encoded in the form of single-stranded, negative-sense RNA. Unlike retroviruses they do not employ DNA intermediates in their life-cycle. Respiratory Syncytial Virus genome Genome The complete genetic complement contained in the DNA of a set of chromosomes in a human. The length of the human genome is about 3 billion base pairs. Basic Terms of Genetics, which is triple-segmented. Infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease are generally either arthropod borne or rodent borne. There are several clinical manifestations, which overall present as hemorrhagic fevers and/or encephalitis Encephalitis Encephalitis is inflammation of the brain parenchyma caused by an infection, usually viral. Encephalitis may present with mild symptoms such as headache, fever, fatigue, and muscle and joint pain or with severe symptoms such as seizures, altered consciousness, and paralysis. Encephalitis. Diagnostic measures include serology Serology The study of serum, especially of antigen-antibody reactions in vitro. Yellow Fever Virus and RT-PCR RT-PCR A variation of the pcr technique in which cDNA is made from RNA via reverse transcription. The resultant cDNA is then amplified using standard pcr protocols. Polymerase Chain Reaction (PCR). Management is largely supportive.
Last updated: Dec 8, 2024
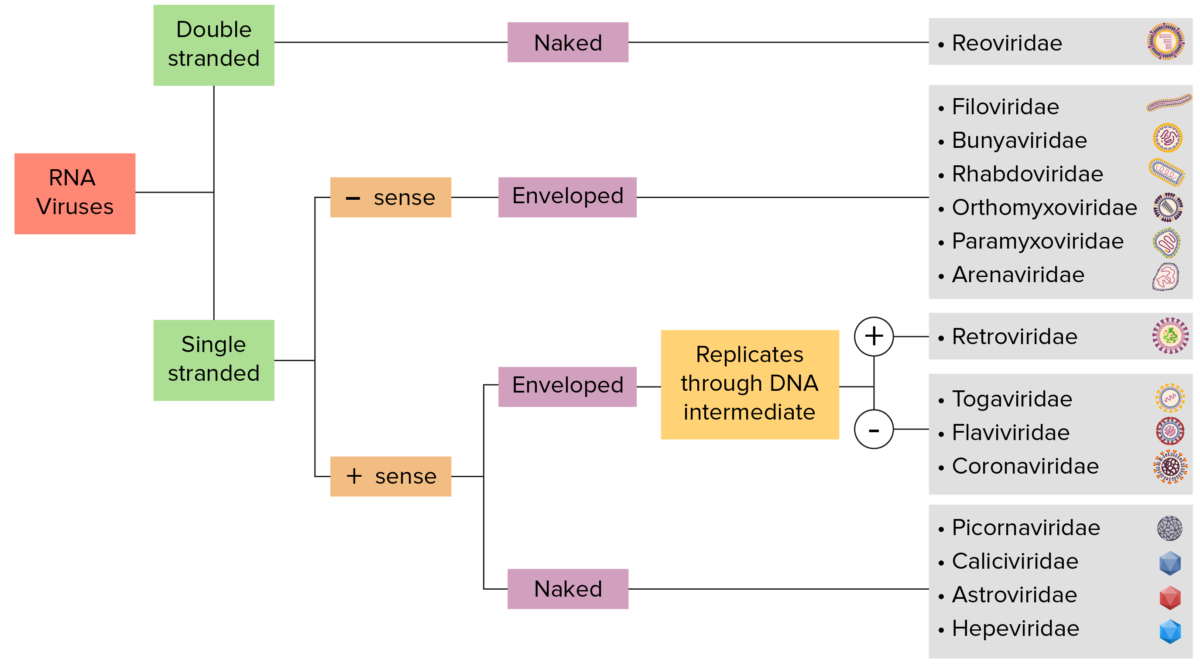
RNA virus identification:
Viruses can be classified in many ways. Most viruses, however, will have a genome formed by either DNA or RNA. RNA genome viruses can be further characterized based on the presence of single- or double-stranded RNA. “Enveloped” viruses are covered by a thin coat of cell membrane (usually taken from the host cell). If the coat is absent, the viruses are called “naked” viruses. Viruses with single-stranded genomes are called “positive-sense” viruses if the genome is directly used as messenger RNA (mRNA), which is translated into proteins. “Negative-sense,” single-stranded viruses use RNA-dependent RNA polymerase, a viral enzyme, to transcribe their genome into messenger RNA.
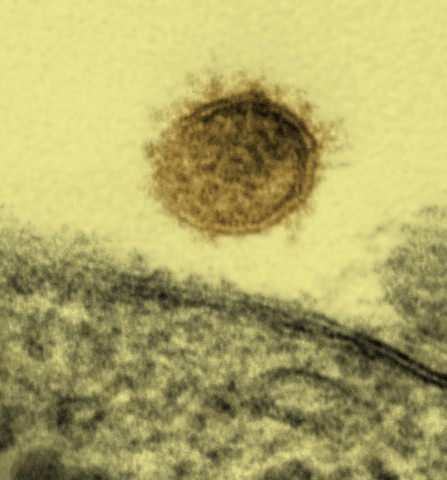
A Sin Nombre virus particle shown budding from a Vero cell:
The Sin Nombre virus causes hantavirus pulmonary syndrome in North America.
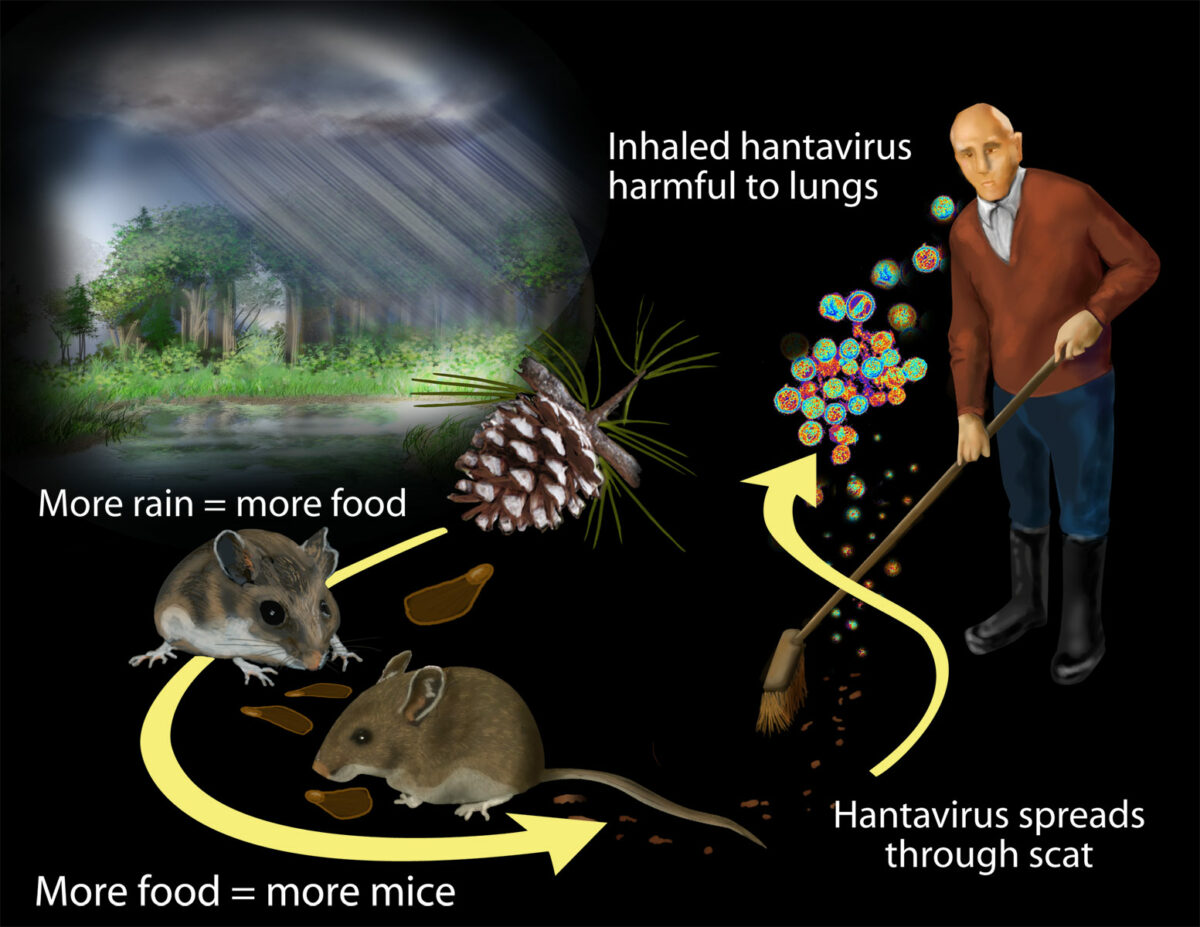
Transmission of hantavirus:
Researchers found that contact with rodents and their waste puts humans at risk for exposure to hantavirus. Massive rainfall associated with the 1991–1992 El Niño boosted plant productivity.
The rodent population grew by feasting on the more abundant plant matter. Increased contact with rodents and their waste put more humans at risk for exposure to hantavirus.
| Hantavirus pulmonary syndrome | Hantavirus hemorrhagic fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever | |
|---|---|---|
| Incubation Incubation The amount time between exposure to an infectious agent and becoming symptomatic. Rabies Virus | 1–3 weeks | 1–3 weeks (up to 6 weeks) |
| Diagnosis |
|
|
| Clinical manifestations | Prodrome Prodrome Symptoms that appear 24–48 hours prior to migraine onset. Migraine Headache: flu-like symptoms Flu-Like Symptoms Babesia/Babesiosis, sudden onset of shortness of breath Shortness of breath Dyspnea is the subjective sensation of breathing discomfort. Dyspnea is a normal manifestation of heavy physical or psychological exertion, but also may be caused by underlying conditions (both pulmonary and extrapulmonary). Dyspnea with rapidly evolving pulmonary edema Pulmonary edema Pulmonary edema is a condition caused by excess fluid within the lung parenchyma and alveoli as a consequence of a disease process. Based on etiology, pulmonary edema is classified as cardiogenic or noncardiogenic. Patients may present with progressive dyspnea, orthopnea, cough, or respiratory failure. Pulmonary Edema | Fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever, low BP/ shock Shock Shock is a life-threatening condition associated with impaired circulation that results in tissue hypoxia. The different types of shock are based on the underlying cause: distributive (↑ cardiac output (CO), ↓ systemic vascular resistance (SVR)), cardiogenic (↓ CO, ↑ SVR), hypovolemic (↓ CO, ↑ SVR), obstructive (↓ CO), and mixed. Types of Shock, AKI AKI Acute kidney injury refers to sudden and often reversible loss of renal function, which develops over days or weeks. Azotemia refers to elevated levels of nitrogen-containing substances in the blood that accompany AKI, which include BUN and creatinine. Acute Kidney Injury |
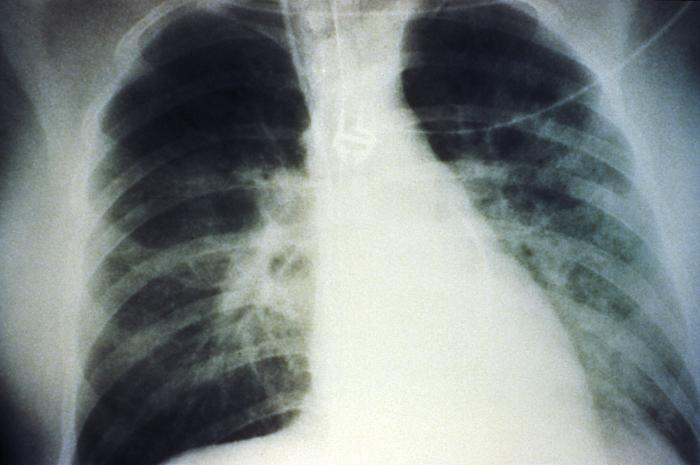
An anteroposterior chest X-ray shows mid-staged bilateral pulmonary effusion due to hantavirus pulmonary syndrome (HPS):
The radiological evolution of HPS begins with minimal changes of interstitial pulmonary edema progressing to alveolar edema with severe bilateral involvement. Pleural effusions are common and often large enough to be evident radiographically.
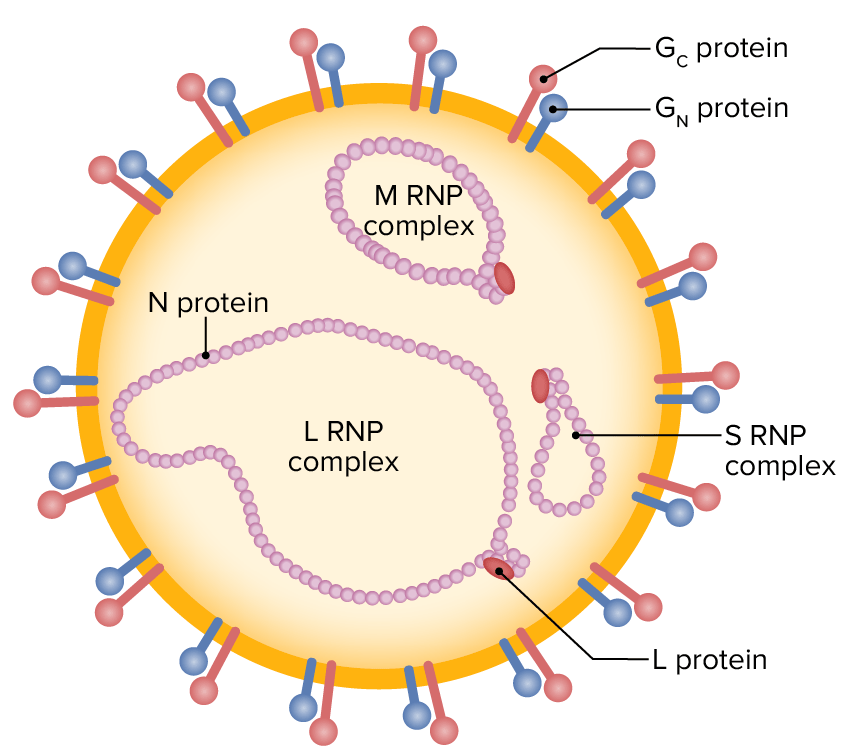
Schematic illustration of a nairovirus particle (enveloped, single-stranded RNA containing L, M, and S segments, surrounded by external glycoproteins)
RNP: ribonucleoprotein
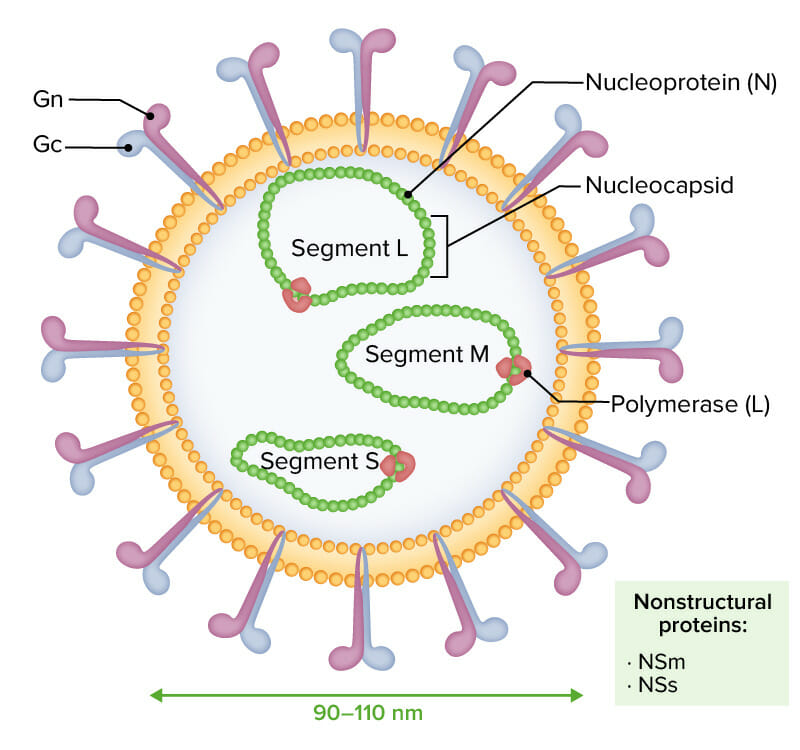
Enveloped virions of Rift Valley fever virus are characterized by a negative or ambisense RNA genome composed of 3 single-stranded segments (designated L, M, and S):
The 3 RNA molecules are encapsidated by nucleoprotein (N), shaping the nucleocapsid and interacting with the viral polymerase (L). Glycoproteins Gn and Gc are noted externally. Nonstructural proteins NSm and NSs are expressed during infection.
Image showing the ultrastructural morphology exhibited by numerous La Crosse virus (LCV) particles
Image: “Image showing the ultrastructural morphology exhibited by numerous La Crosse virus (LCV) particles” by Dr. Erskine Palmer, USCDCP. License: Public Domain| Organism | Hantavirus | Crimean-Congo hemorrhagic fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology | Rift Valley fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology | La Crosse virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology | |
|---|---|---|---|---|---|
| Characteristics | Single-stranded, enveloped, negative-sense RNA Negative-sense RNA RNA viruses that have their genetic material encoded in the form of single-stranded, negative-sense RNA. Unlike retroviruses they do not employ DNA intermediates in their life-cycle. Respiratory Syncytial Virus viruses Viruses Minute infectious agents whose genomes are composed of DNA or RNA, but not both. They are characterized by a lack of independent metabolism and the inability to replicate outside living host cells. Virology | ||||
| Genus | Hantavirus or Orthohantavirus | Nairovirus | Phlebovirus | Orthobunyvirus | |
| Transmission | Inhalation and direct contact with the urine and feces of infected mice | Contact with infected ticks Ticks Blood-sucking acarid parasites of the order ixodida comprising two families: the softbacked ticks (argasidae) and hardbacked ticks (ixodidae). Ticks are larger than their relatives, the mites. They penetrate the skin of their host by means of highly specialized, hooked mouth parts and feed on its blood. Ticks attack all groups of terrestrial vertebrates. In humans they are responsible for many tick-borne diseases, including the transmission of rocky mountain spotted fever; tularemia; babesiosis; african swine fever; and relapsing fever. Coxiella/Q Fever (especially Hyalomma spp.) |
|
Mosquito bites | |
| Clinical presentation |
|
Hemorrhagic fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever |
|
Encephalitis Encephalitis Encephalitis is inflammation of the brain parenchyma caused by an infection, usually viral. Encephalitis may present with mild symptoms such as headache, fever, fatigue, and muscle and joint pain or with severe symptoms such as seizures, altered consciousness, and paralysis. Encephalitis | |
| Diagnosis |
|
|
|
|
|
| Management | Supportive |
|
Supportive | Supportive | |