Cervical cancer is the 3rd most common gynecologic cancer. More than 90% of cervical cancer cases are associated with high-risk human papillomavirus (hrHPV), which is transmitted by sexual contact. Cervical cancer can be prevented by early detection and treatment of precancerous lesions caused by hrHPV. The methods of detection are cervical cytology and HPV testing. Guidelines vary on when to start screening, with various US societies recommending that screening start between 21 and 25 years of age, while the World Health Organization (WHO) suggests waiting until age 30, especially in resource-limited settings. Guidelines also vary on the preferred method of testing, though HPV testing (with or without cytology) is universally preferred starting at age 30. Since the screening program was initiated, there has been a 75% decline in the incidence of and mortality from cervical cancer.
Last updated: Feb 9, 2023
Screening Screening Preoperative Care strategies can be done independently or concurrently (co-testing).
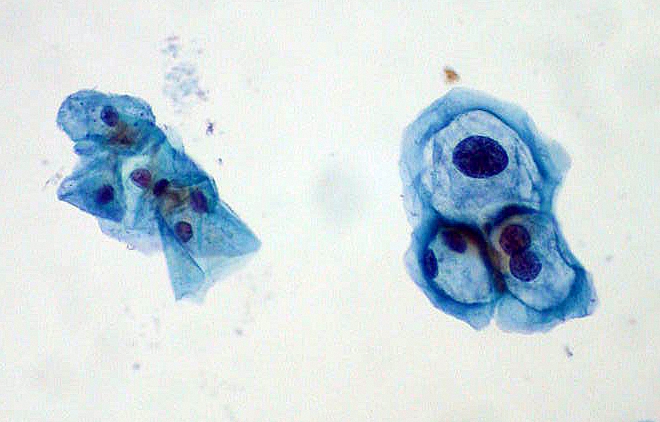
Slide image from a Pap smear for cervical cancer screening: Normal squamous cells are on the left; HPV-infected cells with mild dysplasia are on the right.
Image: “ThinPrep Pap smear HPV” by Ed Uthman. License: Public Domain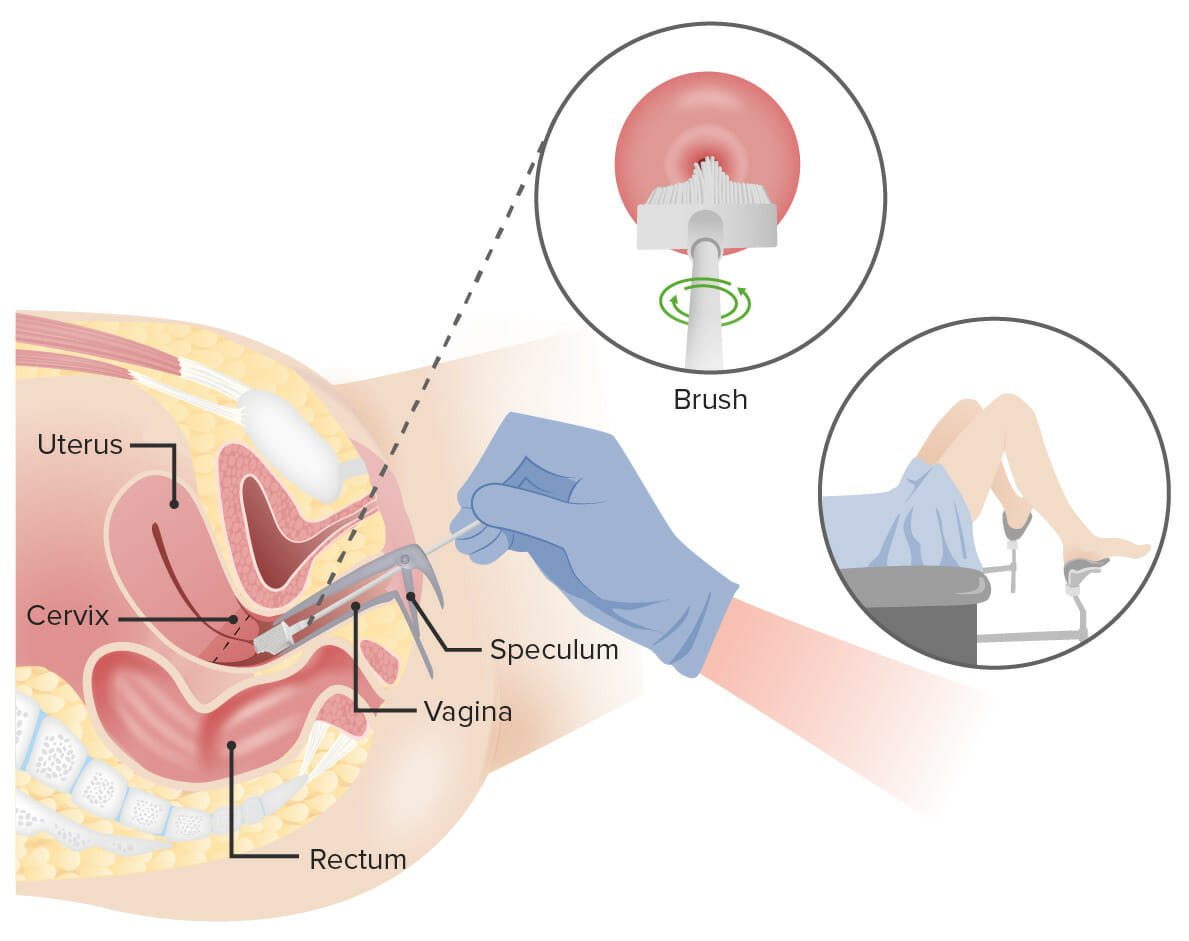
Pap test:
A speculum is inserted into the vagina to widen it. Then, a broom (shown here) or an endocervical brush and spatula is/are inserted into the vagina to collect cells from the cervix. The cells are checked under a microscope for signs of disease.
The following recommendations are for average-risk individuals. This group includes individuals who are fully vaccinated against HPV HPV Human papillomavirus (HPV) is a nonenveloped, circular, double-stranded DNA virus belonging to the Papillomaviridae family. Humans are the only reservoir, and transmission occurs through close skin-to-skin or sexual contact. Human papillomaviruses infect basal epithelial cells and can affect cell-regulatory proteins to result in cell proliferation. Papillomavirus (HPV).
Certain conditions have a high risk for cervical cancer Cervical cancer Cervical cancer, or invasive cervical carcinoma (ICC), is the 3rd most common cancer in women in the world, with > 50% of the cases being fatal. In the United States, ICC is the 13th most common cancer and the cause of < 3% of all cancer deaths due to the slow progression of precursor lesions and, more importantly, effective cancer screening. Cervical Cancer, so screening Screening Preoperative Care must be individualized and more frequent:
The Bethesda system is a standardized reporting of results, which includes the specimen adequacy, general categorization Categorization Types of Variables of findings, and results.
Types of squamous cell epithelial cell abnormalities:
Glandular cell abnormalities (categorized as endocervical, endometrial, or not otherwise specified):
Other possible findings reported on cervical cytology reports:
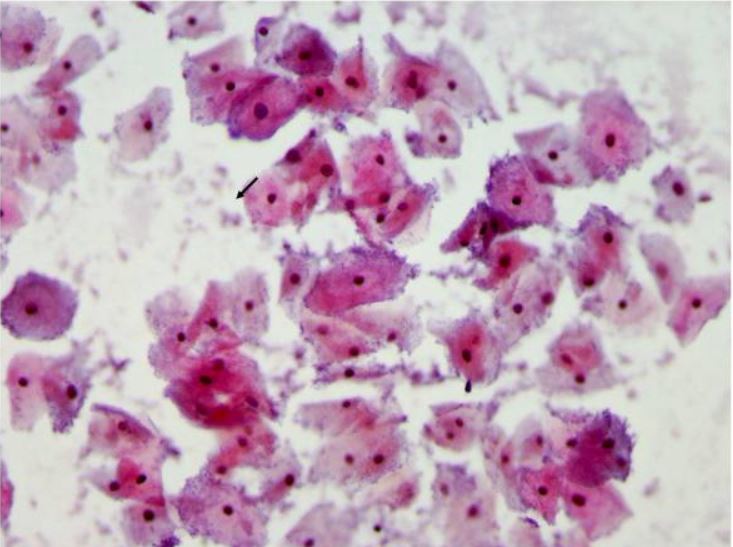
Pap smear showing bacterial vaginosis with many clue cells
Clue cells are vaginal epithelial cells studded with adherent coccobacilli that are best appreciated at the edge of the cells. The bacteria are stained blue-purple by the Pap stain (arrows).
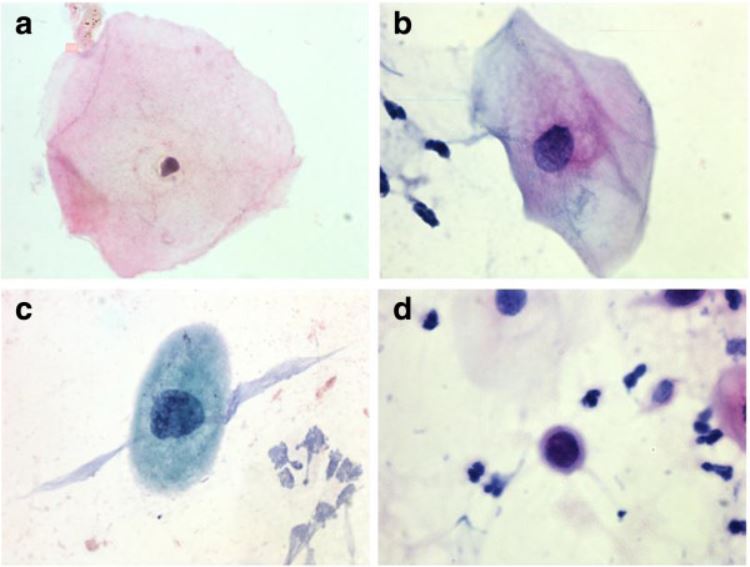
Examples of squamous cell findings during cervical cancer screening:
a. normal cell
b. ASC-US
c. LSIL
d. HSIL
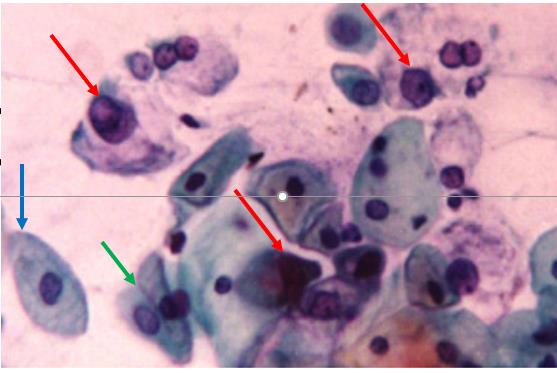
AGCUS favoring a neoplastic process, Pap stain
Cervical cytology showing atypical glandular cells (red arrows), favoring a neoplastic process. The blue arrow points to a benign squamous epithelial cell, and the green arrow points to 2 benign endocervical cells.
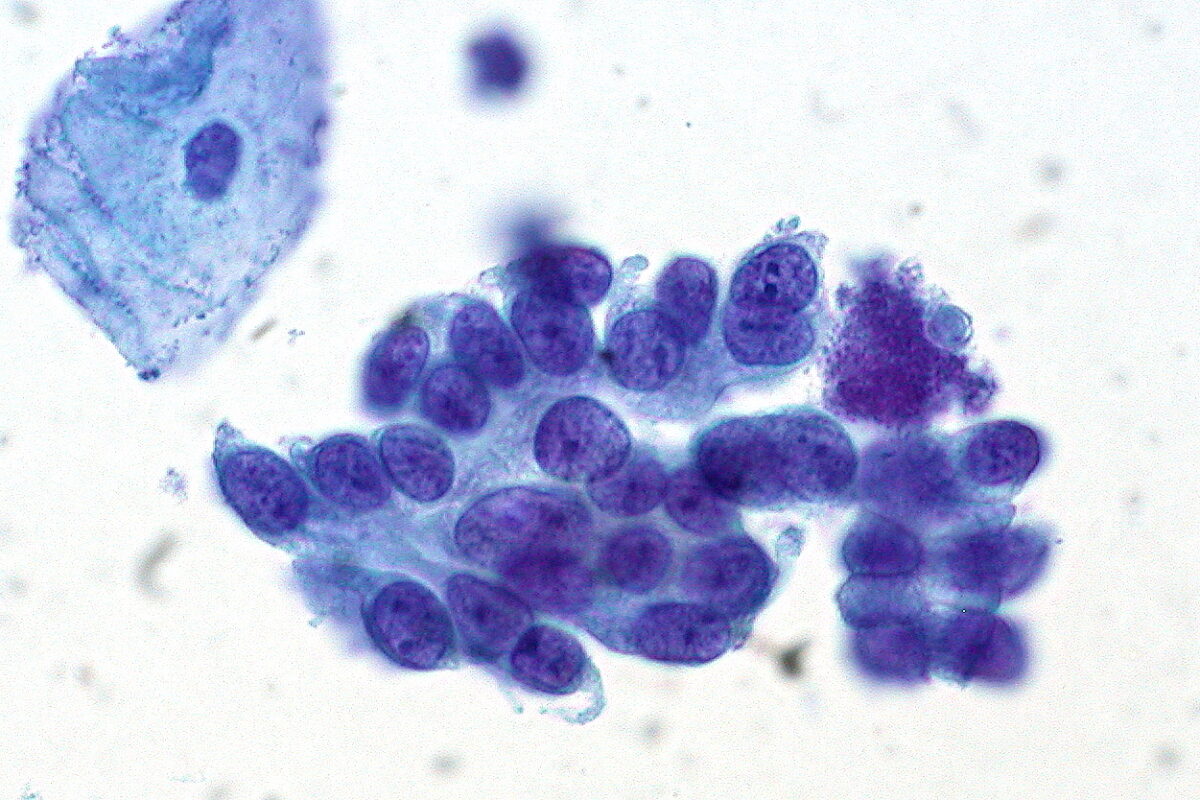
Cervical cytology showing adenocarcinoma in situ of the cervix: Note the benign squamous epithelial cell in the top left.
Image: “Adenocarcinoma in Situ of the Cervix” by Ed Uthman. License: CC BY 2.0Management of abnormal screening Screening Preoperative Care results is based on a patient’s risk of CIN CIN An increased tendency to acquire chromosome aberrations when various processes involved in chromosome replication, repair, or segregation are dysfunctional. Colorectal Cancer 3+ (given as a percentage) both now and in 5 years.
| Risk | Preferred management |
|---|---|
| Risk of CIN CIN An increased tendency to acquire chromosome aberrations when various processes involved in chromosome replication, repair, or segregation are dysfunctional. Colorectal Cancer 3+ developing within the next 5 years (clinical action thresholds) | |
| < 0.15 % | Return to routine screening Screening Preoperative Care at 5-year intervals. |
| 0.15 % to < 0.55 % | Repeat testing (primary HPV HPV Human papillomavirus (HPV) is a nonenveloped, circular, double-stranded DNA virus belonging to the Papillomaviridae family. Humans are the only reservoir, and transmission occurs through close skin-to-skin or sexual contact. Human papillomaviruses infect basal epithelial cells and can affect cell-regulatory proteins to result in cell proliferation. Papillomavirus (HPV) testing, co-testing) in 3 years. |
| 0.55 % to < 4 % | Repeat testing in 1 year. |
| Immediate risk of CIN CIN An increased tendency to acquire chromosome aberrations when various processes involved in chromosome replication, repair, or segregation are dysfunctional. Colorectal Cancer 3+ (clinical action thresholds) | |
| 4 % to < 25 % | Colposcopy recommended |
| 25 % to < 60 % | Expedited treatment or colposcopy acceptable (for nonpregnant patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship ≥ 25 years of age) |
| ≥ 60 % | Expedited treatment preferred (for nonpregnant patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship ≥ 25 years of age) |
| History | Current HPV HPV Human papillomavirus (HPV) is a nonenveloped, circular, double-stranded DNA virus belonging to the Papillomaviridae family. Humans are the only reservoir, and transmission occurs through close skin-to-skin or sexual contact. Human papillomaviruses infect basal epithelial cells and can affect cell-regulatory proteins to result in cell proliferation. Papillomavirus (HPV) result | Current cytology result | CIN CIN An increased tendency to acquire chromosome aberrations when various processes involved in chromosome replication, repair, or segregation are dysfunctional. Colorectal Cancer 3+ immediate risk (%) | CIN CIN An increased tendency to acquire chromosome aberrations when various processes involved in chromosome replication, repair, or segregation are dysfunctional. Colorectal Cancer 3+ 5-year risk (%) | Recommended management |
|---|---|---|---|---|---|
| Unknown | HPV-negative | NILM | 0.00 | 0.12 | 5 years of follow-up |
| ASC-US | 0.04 | 0.40 | 3 years of follow-up | ||
| LSIL | 1.1 | 2.0 | 1 year of follow-up | ||
| ASC-H | 3.4 | 3.8 | Colposcopy | ||
| HSIL+ | 25 | 27 | Colposcopy or expedited treatment | ||
| HPV-positive | NILM | 2.1 | 4.8 | 1 year of follow-up | |
| ASC-US | 4.4 | 7.3 | Colposcopy | ||
| LSIL | 4.3 | 6.9 | Colposcopy | ||
| ASC-H | 26 | 33 | Colposcopy or expedited treatment | ||
| HSIL+ | 49 | 53 | Colposcopy or expedited treatment | ||
| HPV-negative | HPV-negative | NILM | 0.00 | 0.09 | 5 years of follow-up |
| ASC-US | 0.01 | 0.36 | 3 years of follow-up | ||
| LSIL | 0.44 | 0.79 | 1 year of follow-up | ||
| ASC-H | 2.8 | 3.3 | Colposcopy | ||
| HSIL+ | 14 | 14 | Colposcopy | ||
| HPV-positive | NILM | 0.74 | 2.3 | 1 year of follow-up | |
| ASC-US | 2.0 | 3.8 | 1 year of follow-up | ||
| LSIL | 2.1 | 3.8 | 1 year of follow-up | ||
| ASC-H | 14 | 18 | Colposcopy | ||
| HSIL+ | 32 | 34 | Colposcopy or expedited treatment |