Alkylating agents are cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell's progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle–independent antineoplastic drugs that work primarily by binding alkyl groups to various parts of DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure. The overall action produces cross-linking of DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure, leading to inhibition of DNA replication DNA replication The entire DNA of a cell is replicated during the S (synthesis) phase of the cell cycle. The principle of replication is based on complementary nucleotide base pairing: adenine forms hydrogen bonds with thymine (or uracil in RNA) and guanine forms hydrogen bonds with cytosine. DNA Replication and DNA damage DNA Damage Injuries to DNA that introduce deviations from its normal, intact structure and which may, if left unrepaired, result in a mutation or a block of DNA replication. These deviations may be caused by physical or chemical agents and occur by natural or unnatural, introduced circumstances. They include the introduction of illegitimate bases during replication or by deamination or other modification of bases; the loss of a base from the DNA backbone leaving an abasic site; single-strand breaks; double strand breaks; and intrastrand (pyrimidine dimers) or interstrand crosslinking. Damage can often be repaired (DNA repair). If the damage is extensive, it can induce apoptosis. DNA Repair Mechanisms. The general effect is cancer ( CA CA Condylomata acuminata are a clinical manifestation of genital HPV infection. Condylomata acuminata are described as raised, pearly, flesh-colored, papular, cauliflower-like lesions seen in the anogenital region that may cause itching, pain, or bleeding. Condylomata Acuminata (Genital Warts)) cell death Cell death Injurious stimuli trigger the process of cellular adaptation, whereby cells respond to withstand the harmful changes in their environment. Overwhelmed adaptive mechanisms lead to cell injury. Mild stimuli produce reversible injury. If the stimulus is severe or persistent, injury becomes irreversible. Apoptosis is programmed cell death, a mechanism with both physiologic and pathologic effects. Cell Injury and Death. The subgroups of drugs are nitrogen Nitrogen An element with the atomic symbol n, atomic number 7, and atomic weight [14. 00643; 14. 00728]. Nitrogen exists as a diatomic gas and makes up about 78% of the earth's atmosphere by volume. It is a constituent of proteins and nucleic acids and found in all living cells. Urea Cycle mustards, nitrosoureas, alkyl sulfonates, triazines, ethylenimines, and methylmelamines. Platinum coordination Coordination Cerebellar Disorders complexes belong to the group of alkylating agents by producing the same effect, but their mechanism is via formation of covalent metal adducts with DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure. Myelosuppression Myelosuppression Oxazolidinones and toxicity Toxicity Dosage Calculation to organ systems such as the kidneys Kidneys The kidneys are a pair of bean-shaped organs located retroperitoneally against the posterior wall of the abdomen on either side of the spine. As part of the urinary tract, the kidneys are responsible for blood filtration and excretion of water-soluble waste in the urine. Kidneys: Anatomy, liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy, and lungs Lungs Lungs are the main organs of the respiratory system. Lungs are paired viscera located in the thoracic cavity and are composed of spongy tissue. The primary function of the lungs is to oxygenate blood and eliminate CO2. Lungs: Anatomy are common adverse reactions.
Last updated: Nov 21, 2022
Alkylating agents are antineoplastic drugs that attach an alkyl group onto DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure to cause cancer ( CA CA Condylomata acuminata are a clinical manifestation of genital HPV infection. Condylomata acuminata are described as raised, pearly, flesh-colored, papular, cauliflower-like lesions seen in the anogenital region that may cause itching, pain, or bleeding. Condylomata Acuminata (Genital Warts)) cell death Cell death Injurious stimuli trigger the process of cellular adaptation, whereby cells respond to withstand the harmful changes in their environment. Overwhelmed adaptive mechanisms lead to cell injury. Mild stimuli produce reversible injury. If the stimulus is severe or persistent, injury becomes irreversible. Apoptosis is programmed cell death, a mechanism with both physiologic and pathologic effects. Cell Injury and Death.
Chemical structure of cyclophosphamide
Image: “Cyclophosphamide structure” by Mysid. License: Public Domain
Chemical structure of carmustine
Image: “Carmustine” by Fvasconcellos. License: Public Domain
Chemical structure of lomustine
Image: “Lomustine” by Fvasconcellos. License: Public Domain
Chemical structure of busulfan
Image: “Busulfan” by Fvasconcellos. License: Public Domain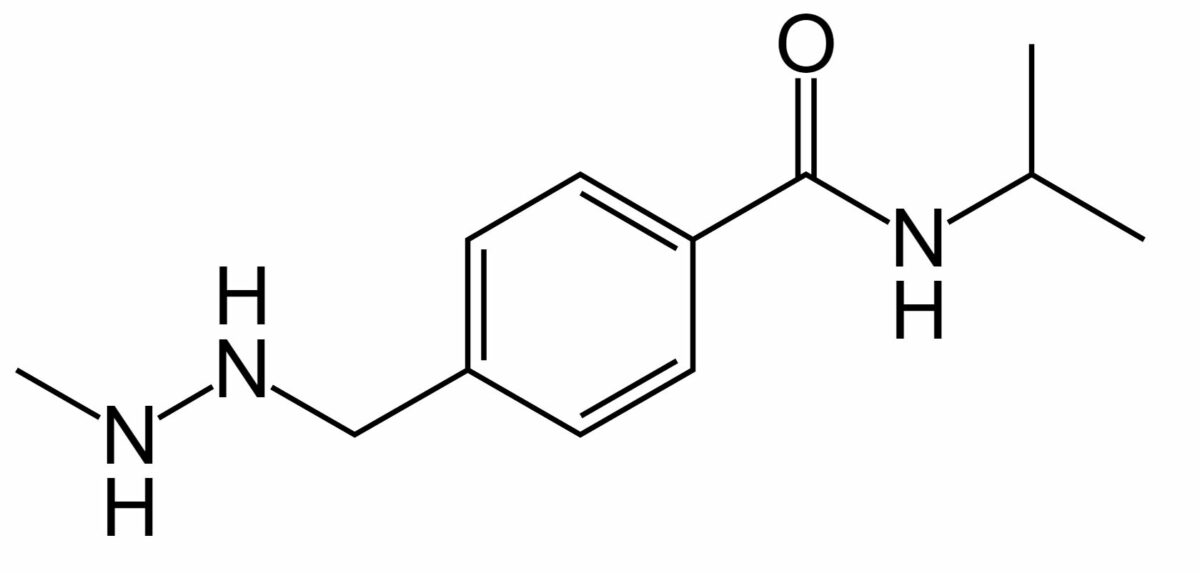
Chemical structure of procarbazine
Image: “Procarbazine” by Fvasconcellos. License: Public Domain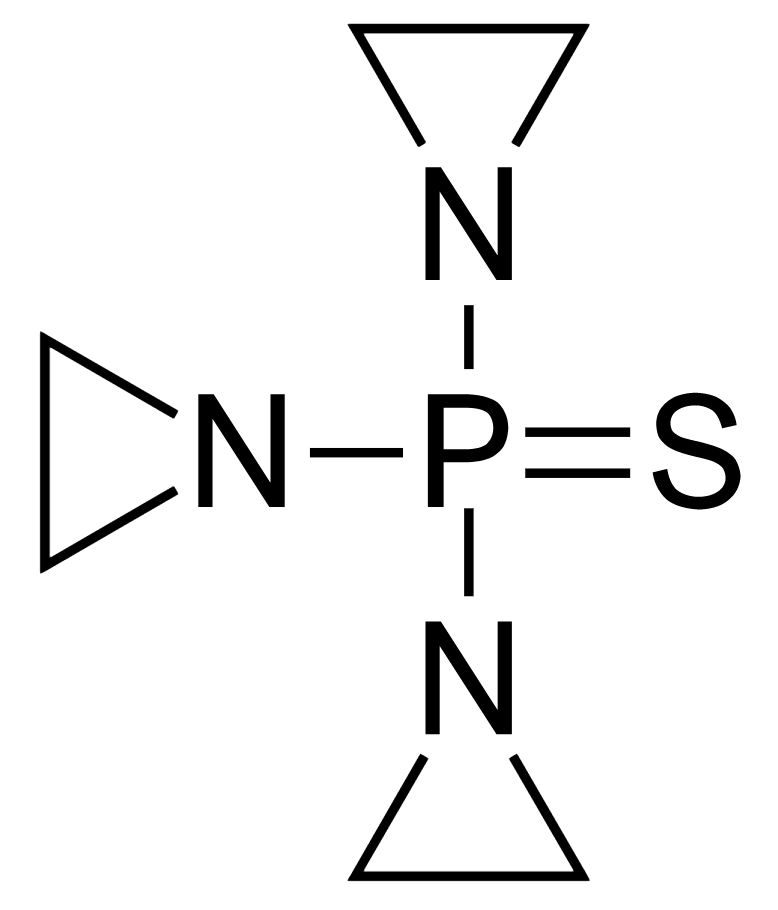
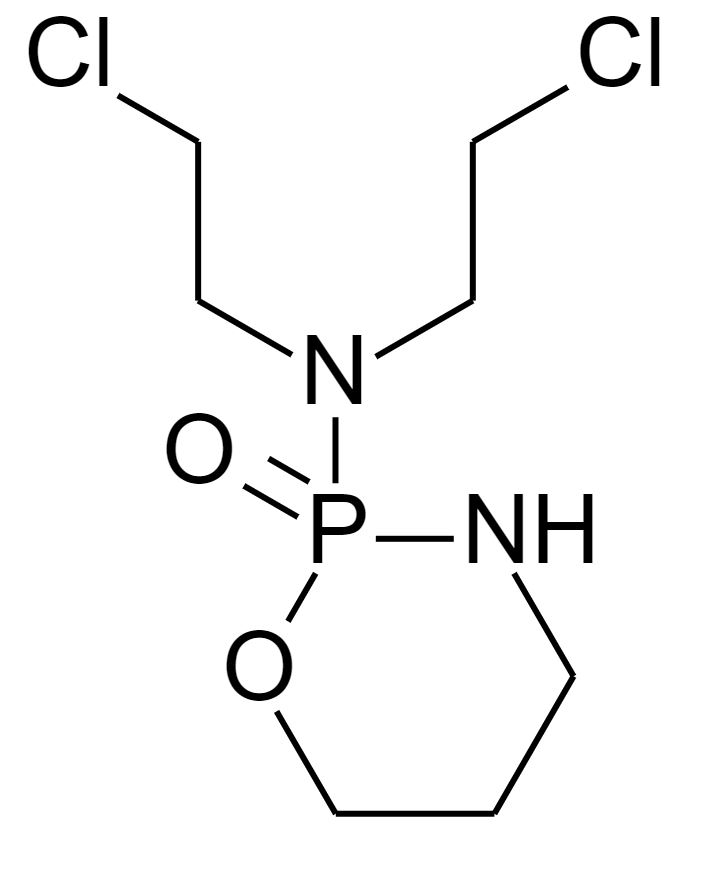
Chemical structure of thiotepa
Image: “ThioTEPA” by Fvasconcellos. License: Public Domain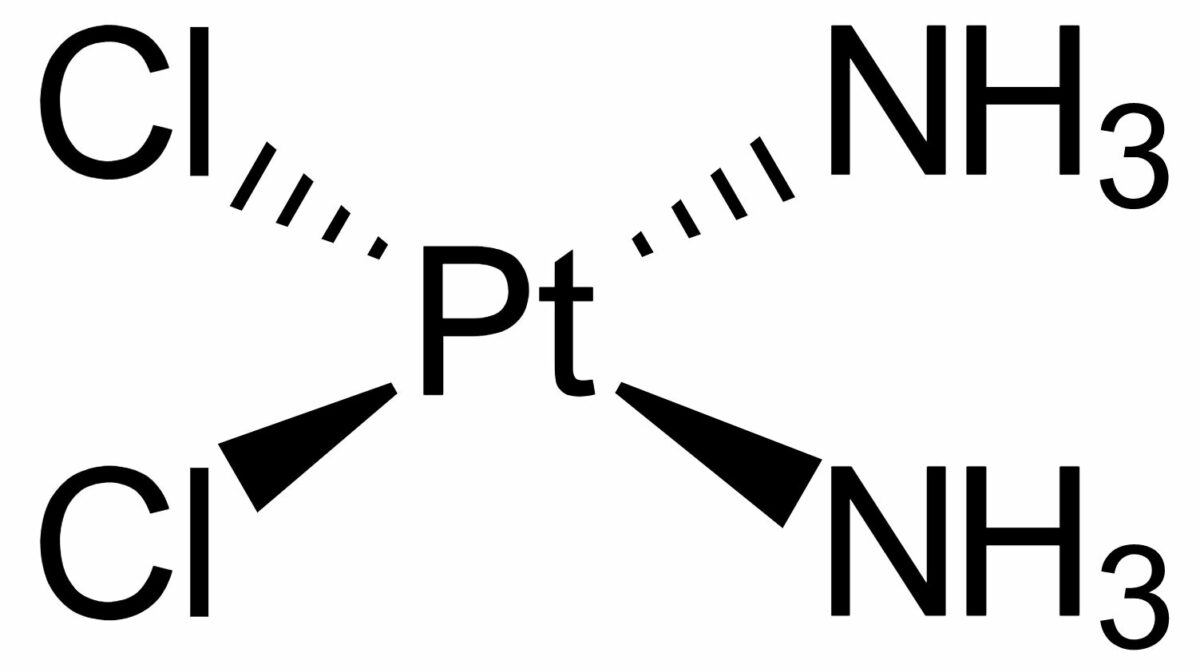
Chemical structure of cisplatin
Image: “Cisplatin-stereo” by Benrr101. License: Public Domain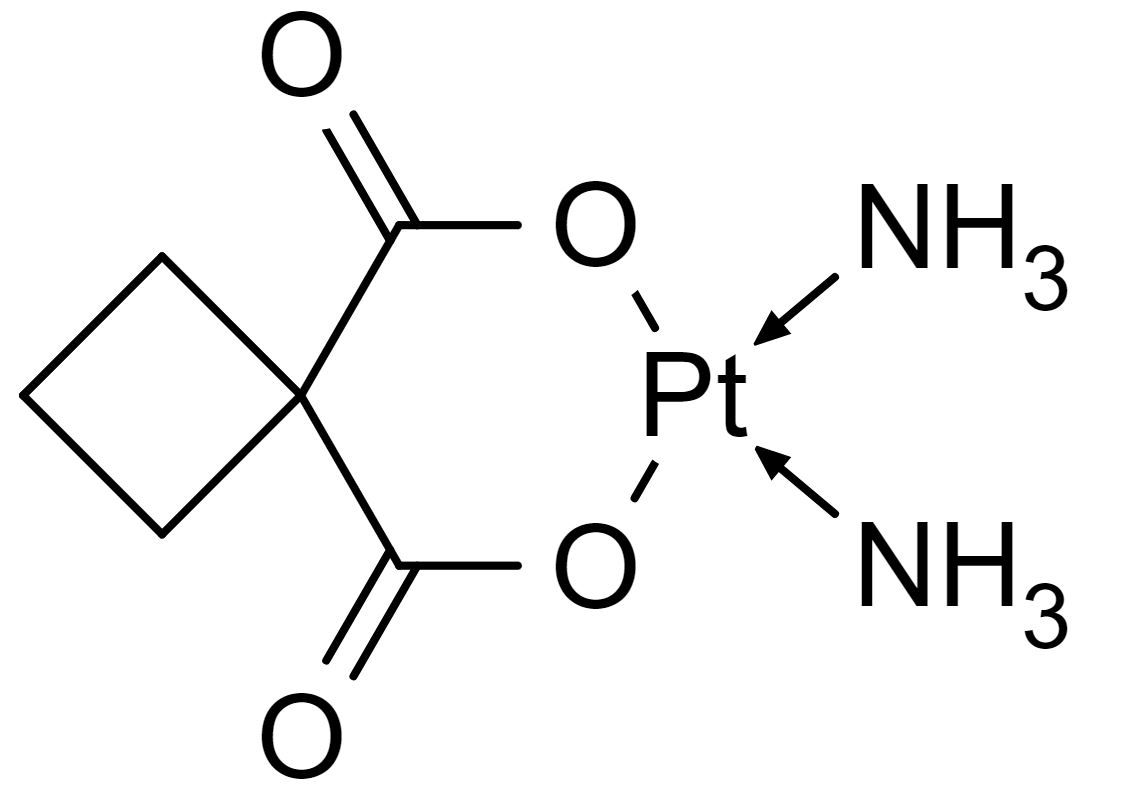
Chemical structure of carboplatin
Image: “Carboplatin-skeletal” by catclock. License: Public Domain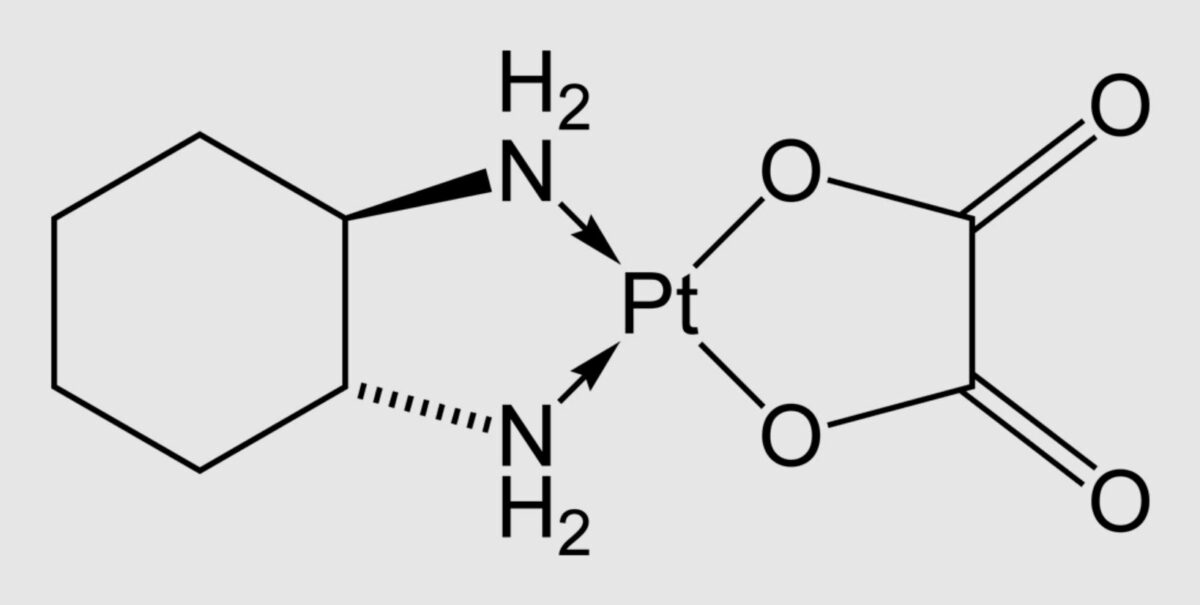
Chemical structure of oxaliplatin
Image: “Oxaliplatin-2D-skeletal” by Benjah-bmm27. License: Public Domain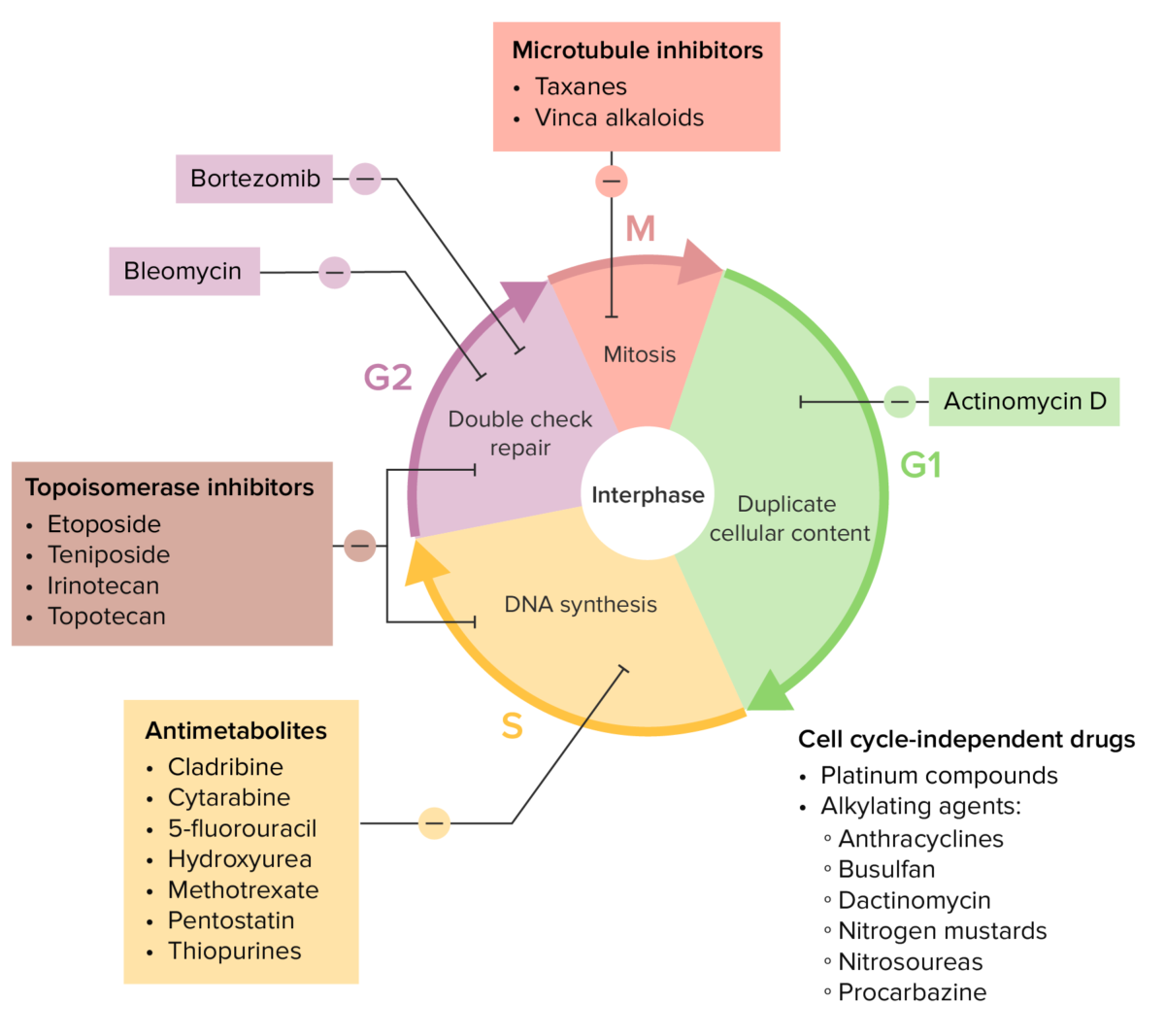
Various chemotherapy drugs and their effects on the cell cycle
Image by Lecturio.| Drug class | Mechanism |
|---|---|
Antitumor antibiotics
Antitumor Antibiotics
Antitumor antibiotics, also known as antineoplastic antibiotics, are the product of soil microbes, Streptomyces bacteria. The commonly used types of antitumor antibiotics (bleomycin, dactinomycin, and anthracyclines) have a wide spectrum of activity against hematologic malignancies and solid tumors.
Antitumor Antibiotics:
|
Intercalation between bases Bases Usually a hydroxide of lithium, sodium, potassium, rubidium or cesium, but also the carbonates of these metals, ammonia, and the amines. Acid-Base Balance leading to blockage of DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure or RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure synthesis Synthesis Polymerase Chain Reaction (PCR), and prevention of DNA replication DNA replication The entire DNA of a cell is replicated during the S (synthesis) phase of the cell cycle. The principle of replication is based on complementary nucleotide base pairing: adenine forms hydrogen bonds with thymine (or uracil in RNA) and guanine forms hydrogen bonds with cytosine. DNA Replication |
| Anthracyclines Anthracyclines Organic compounds that have a tetrahydronaphthacenedione ring structure attached by a glycosidic linkage to the amino sugar daunosamine. Antitumor Antibiotics |
|
| Alkylating agents |
|