Glioblastoma multiforme is a high-grade astrocytoma Astrocytoma Astrocytomas are neuroepithelial tumors that arise from astrocytes, which are star-shaped glial cells (supporting tissues of the CNS). Astrocytomas are a type of glioma. There are 4 grades of astrocytomas. Astrocytoma, an aggressive brain Brain The part of central nervous system that is contained within the skull (cranium). Arising from the neural tube, the embryonic brain is comprised of three major parts including prosencephalon (the forebrain); mesencephalon (the midbrain); and rhombencephalon (the hindbrain). The developed brain consists of cerebrum; cerebellum; and other structures in the brain stem. Nervous System: Anatomy, Structure, and Classification tumor Tumor Inflammation arising from astrocytes Astrocytes A class of large neuroglial (macroglial) cells in the central nervous system - the largest and most numerous neuroglial cells in the brain and spinal cord. Astrocytes (from 'star' cells) are irregularly shaped with many long processes, including those with 'end feet' which form the glial (limiting) membrane and directly and indirectly contribute to the blood-brain barrier. They regulate the extracellular ionic and chemical environment, and 'reactive astrocytes' (along with microglia) respond to injury. Nervous System: Histology, with an unknown cause and a poorly understood link to risk factors. There are two main types: primary, a more aggressive form seen more commonly in older patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship, and secondary, developing from lower-grade astrocytomas and seen more commonly in younger patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship. Glioblastomas most commonly present with headache Headache The symptom of pain in the cranial region. It may be an isolated benign occurrence or manifestation of a wide variety of headache disorders. Brain Abscess, seizures Seizures A seizure is abnormal electrical activity of the neurons in the cerebral cortex that can manifest in numerous ways depending on the region of the brain affected. Seizures consist of a sudden imbalance that occurs between the excitatory and inhibitory signals in cortical neurons, creating a net excitation. The 2 major classes of seizures are focal and generalized. Seizures, and neurologic deficits Neurologic Deficits High-Risk Headaches. MRI is the gold standard diagnostic tool, and surgical resection combined with radiation Radiation Emission or propagation of acoustic waves (sound), electromagnetic energy waves (such as light; radio waves; gamma rays; or x-rays), or a stream of subatomic particles (such as electrons; neutrons; protons; or alpha particles). Osteosarcoma and chemotherapy Chemotherapy Osteosarcoma is the treatment of choice. Prognosis Prognosis A prediction of the probable outcome of a disease based on a individual's condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas is extremely poor, with survival of only 1–5 years in patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship receiving aggressive treatment and only 3 months in patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship who do not undergo treatment.
Last updated: May 17, 2024
Glioblastoma multiforme (GBM) is an aggressive, rapidly progressive type of brain Brain The part of central nervous system that is contained within the skull (cranium). Arising from the neural tube, the embryonic brain is comprised of three major parts including prosencephalon (the forebrain); mesencephalon (the midbrain); and rhombencephalon (the hindbrain). The developed brain consists of cerebrum; cerebellum; and other structures in the brain stem. Nervous System: Anatomy, Structure, and Classification tumor Tumor Inflammation arising from astrocytes Astrocytes A class of large neuroglial (macroglial) cells in the central nervous system – the largest and most numerous neuroglial cells in the brain and spinal cord. Astrocytes (from ‘star’ cells) are irregularly shaped with many long processes, including those with ‘end feet’ which form the glial (limiting) membrane and directly and indirectly contribute to the blood-brain barrier. They regulate the extracellular ionic and chemical environment, and ‘reactive astrocytes’ (along with microglia) respond to injury. Nervous System: Histology:
| Categories | Specific tumors |
|---|---|
| Neuroepithelial tumors in the CNS |
|
| Meningeal tumors |
|
| Sellar region tumors |
|
| Primary CNS lymphoma Lymphoma A general term for various neoplastic diseases of the lymphoid tissue. Imaging of the Mediastinum | Primary CNS lymphoma Lymphoma A general term for various neoplastic diseases of the lymphoid tissue. Imaging of the Mediastinum |
| Metastasis Metastasis The transfer of a neoplasm from one organ or part of the body to another remote from the primary site. Grading, Staging, and Metastasis to the brain Brain The part of central nervous system that is contained within the skull (cranium). Arising from the neural tube, the embryonic brain is comprised of three major parts including prosencephalon (the forebrain); mesencephalon (the midbrain); and rhombencephalon (the hindbrain). The developed brain consists of cerebrum; cerebellum; and other structures in the brain stem. Nervous System: Anatomy, Structure, and Classification (5× more common than primary brain Brain The part of central nervous system that is contained within the skull (cranium). Arising from the neural tube, the embryonic brain is comprised of three major parts including prosencephalon (the forebrain); mesencephalon (the midbrain); and rhombencephalon (the hindbrain). The developed brain consists of cerebrum; cerebellum; and other structures in the brain stem. Nervous System: Anatomy, Structure, and Classification tumors) | Most commonly arising from: |
| Peripheral tumors |
|
The 2 main types of glioblastoma are:
The cause of glioblastomas is unclear and it is difficult to determine a single cause. The risk factors that appear to contribute to glioblastoma include:
Several genetic mutations Genetic Mutations Carcinogenesis are associated with astrocytomas:
Many generalized symptoms are due to increases in ICP ICP Normal intracranial pressure (ICP) is defined as < 15 mm Hg, whereas pathologically increased ICP is any pressure ≥ 20 mm Hg. Increased ICP may result from several etiologies, including trauma, intracranial hemorrhage, mass lesions, cerebral edema, increased CSF production, and decreased CSF absorption. Increased Intracranial Pressure (ICP). Symptoms may include:
Diagnosis of glioblastomas is based mainly on imaging after the clinical presentation and a careful history and exam raise suspicion for a brain Brain The part of central nervous system that is contained within the skull (cranium). Arising from the neural tube, the embryonic brain is comprised of three major parts including prosencephalon (the forebrain); mesencephalon (the midbrain); and rhombencephalon (the hindbrain). The developed brain consists of cerebrum; cerebellum; and other structures in the brain stem. Nervous System: Anatomy, Structure, and Classification tumor Tumor Inflammation. A biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma is required to confirm the diagnosis. Laboratory studies are not helpful in diagnosing GBMs.
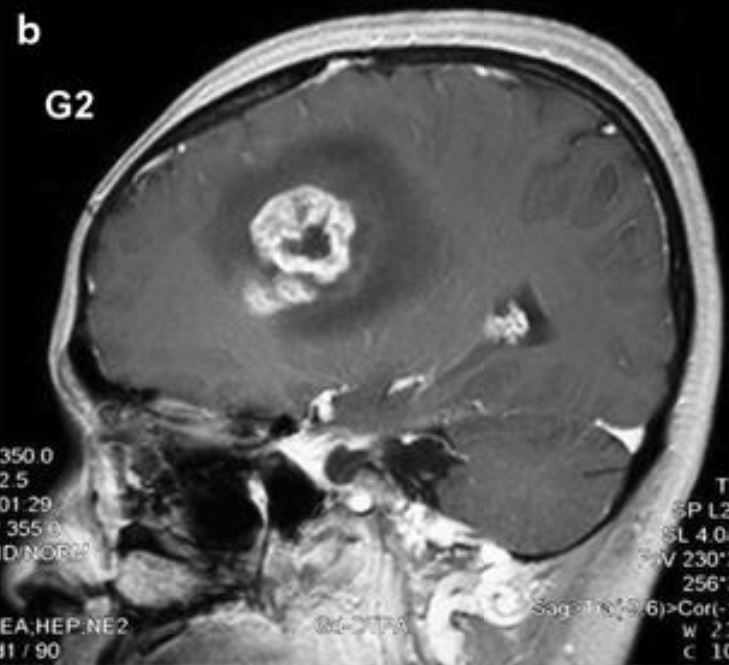
MRI scan of a glioblastoma:
Enhancement of the lesion with central clearing indicates central necrosis.
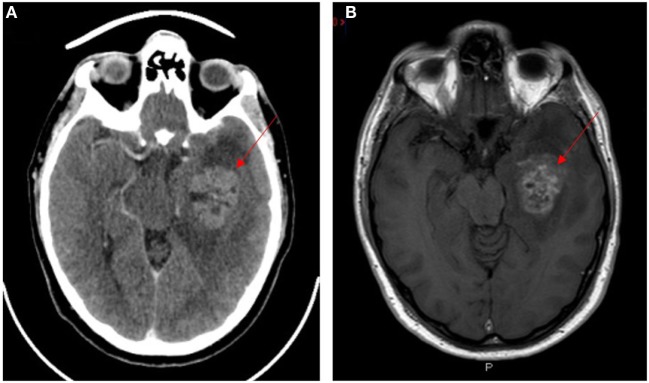
Neuroimaging of glioblastoma multiforme (lesions indicated by red arrows):
A: Contrast-enhanced CT
B: T1-weighted contrast-enhanced MRI
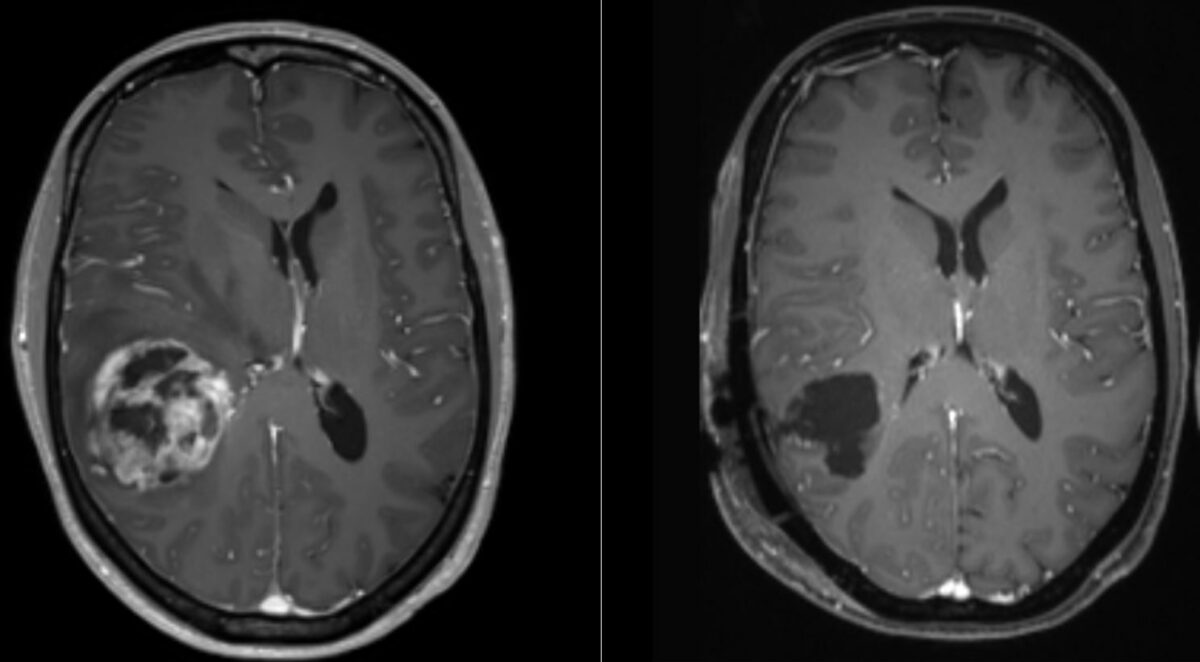
MRI of glioblastoma:
The image represents the tumor seen preoperatively (left) and postoperatively (right). The image on the left shows a central area of necrosis surrounded by an enhanced region and a midline shift.
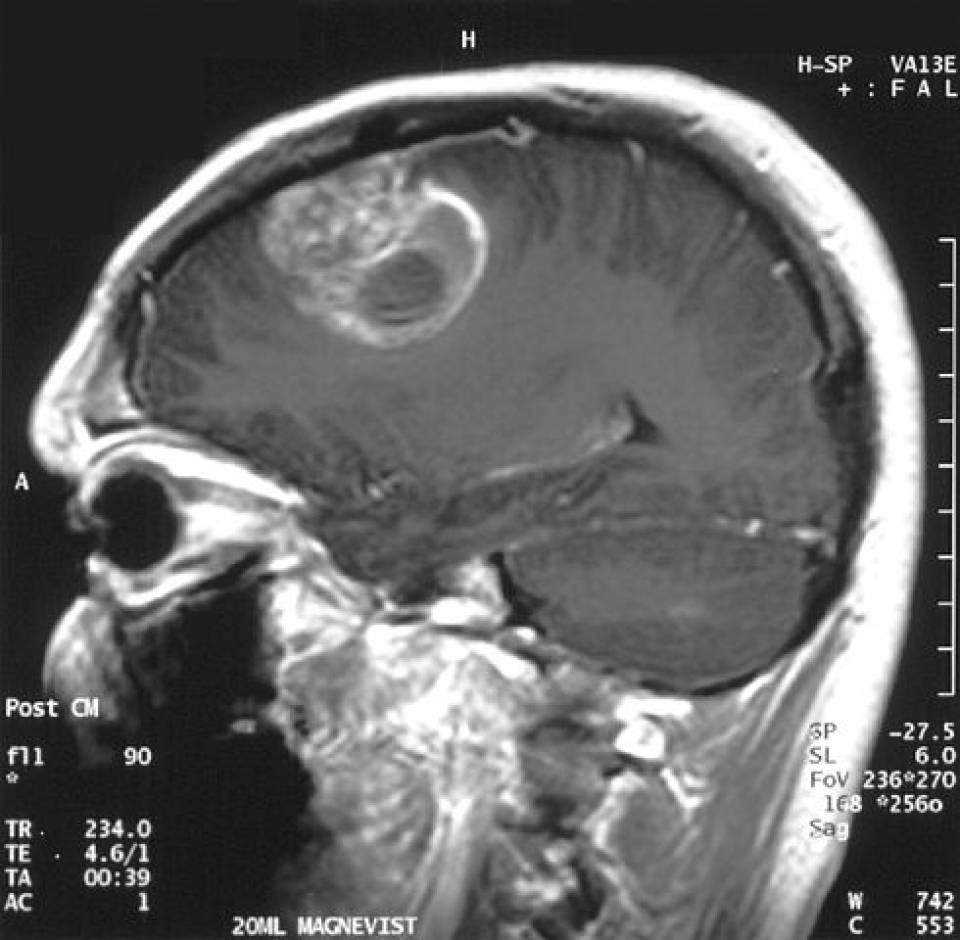
Sagittal MRI showing a mass in the brain diagnosed as glioblastoma:
The lesion is surrounded by an area of enhancement and a central area of necrosis.
A histopathologic specimen obtained through biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma is required for definitive diagnosis. Findings include:
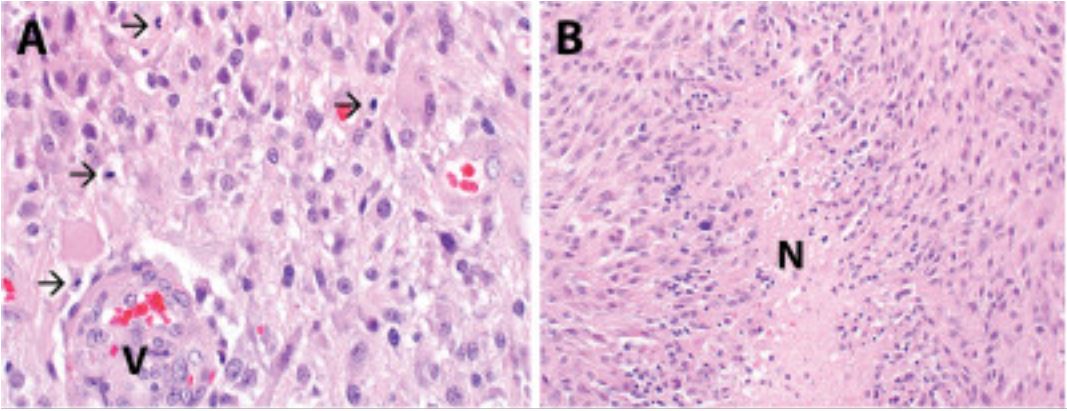
Glioblastoma multiforme (WHO grade IV astrocytoma):
Histopathologic evaluation reveals a hypercellular astrocytic neoplasm infiltrating the surrounding brain parenchyma.
B: Necrosis is seen in the specimen, including pseudopalisading necrosis (designated N).
Image: “Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy” by Robinson GW, Orr BA, Gajjar A. License: CC BY 2.0, cropped by Lecturio.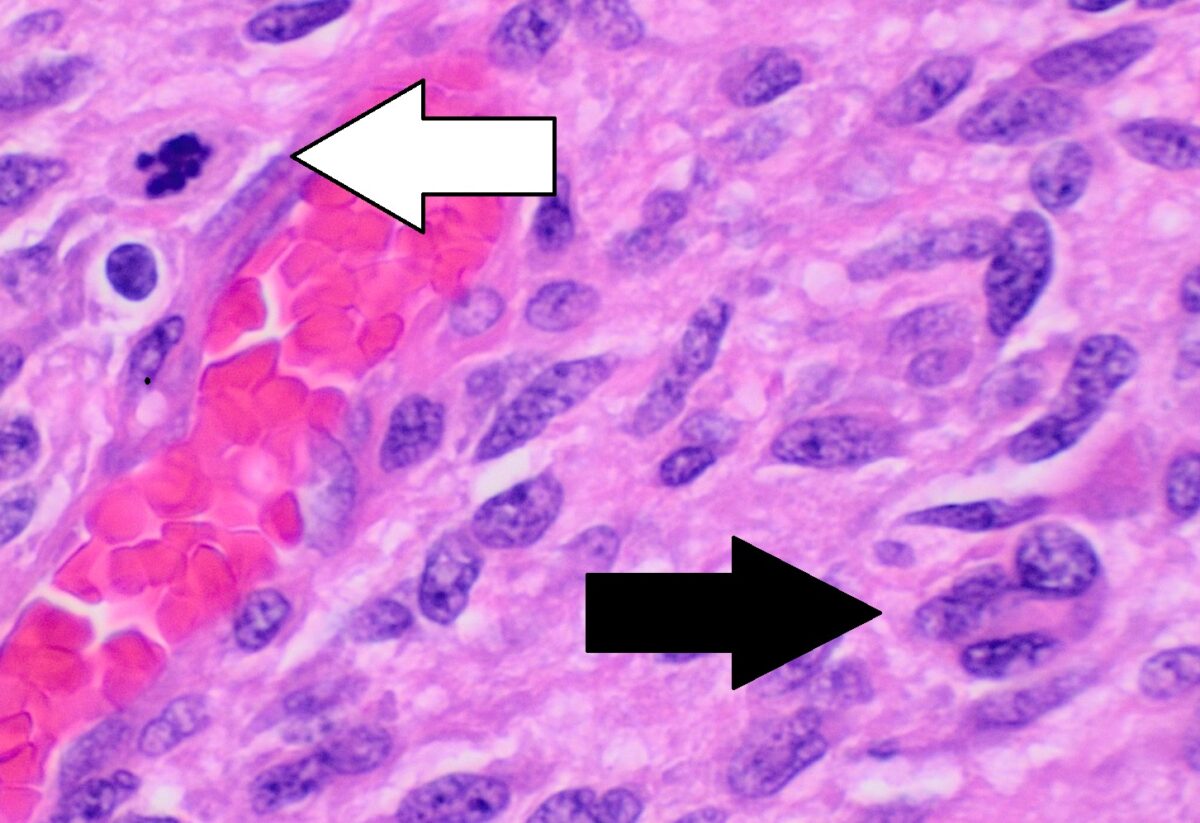
Histopathology of glioblastoma showing nuclear pleomorphism, multiple mitotic figures (white arrow), and multinucleated cells (black arrow)
There is a patternless arrangement of cells.
The main approach to management is surgical intervention, followed by radiation Radiation Emission or propagation of acoustic waves (sound), electromagnetic energy waves (such as light; radio waves; gamma rays; or x-rays), or a stream of subatomic particles (such as electrons; neutrons; protons; or alpha particles). Osteosarcoma and chemotherapy Chemotherapy Osteosarcoma. No treatment is curative, and prognosis Prognosis A prediction of the probable outcome of a disease based on a individual’s condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas remains poor, even with aggressive treatment.
Challenges that hinder the management of glioblastomas are:
For patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship who present with neurologic findings, the following conditions should be considered in the differential diagnosis for glioblastoma multiforme.