Pancreatic cancer, consisting mostly of invasive pancreatic ductal adenocarcinoma Pancreatic Ductal Adenocarcinoma Chronic Pancreatitis (PDAC), arises from the ductal cells Ductal cells Gastrointestinal Secretions of the exocrine pancreas Exocrine pancreas The major component (about 80%) of the pancreas composed of acinar functional units of tubular and spherical cells. The acinar cells synthesize and secrete several digestive enzymes such as trypsinogen; lipase; amylase; and ribonuclease. Secretion from the exocrine pancreas drains into the pancreatic ductal system and empties into the duodenum. Pancreas: Anatomy and is the 4th leading cause of cancer-related deaths in the United States. Pancreatic cancer has the highest mortality Mortality All deaths reported in a given population. Measures of Health Status rate among the major cancers, with a 5-year survival rate of only 8%–10%. Clinical presentation includes symptoms of abdominal pain Abdominal Pain Acute Abdomen, jaundice Jaundice Jaundice is the abnormal yellowing of the skin and/or sclera caused by the accumulation of bilirubin. Hyperbilirubinemia is caused by either an increase in bilirubin production or a decrease in the hepatic uptake, conjugation, or excretion of bilirubin. Jaundice, and weight loss Weight loss Decrease in existing body weight. Bariatric Surgery. Diagnosis is made by CT, MRI, and endoscopic ultrasonography (EUS). Management by surgical resection, usually with neoadjuvant or adjuvant Adjuvant Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (freund's adjuvant, bcg, corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. Vaccination chemotherapy Chemotherapy Osteosarcoma, provides the only chance for cure in the 15%–20% of patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship who have resectable disease at the time of diagnosis. Other rare malignant tumors arising from the exocrine pancreas Exocrine pancreas The major component (about 80%) of the pancreas composed of acinar functional units of tubular and spherical cells. The acinar cells synthesize and secrete several digestive enzymes such as trypsinogen; lipase; amylase; and ribonuclease. Secretion from the exocrine pancreas drains into the pancreatic ductal system and empties into the duodenum. Pancreas: Anatomy are acinar cell carcinoma and pancreatoblastoma.
Last updated: May 17, 2024
| Hereditary/genetic factors | Germ-line mutations | Maximum increased lifetime risk (approximate) |
|---|---|---|
| Peutz-Jeghers syndrome Peutz-Jeghers Syndrome Peutz-Jeghers syndrome (PJS) is an autosomal-dominant inherited disorder characterized by GI polyps and mucocutaneous-pigmented macules. Peutz-Jeghers syndrome is 1 of the polyposis syndromes, a group of inherited or acquired conditions characterized by the growth of polyps in the GI tract and associated with other extracolonic features. Peutz-Jeghers Syndrome ( PJS PJS Peutz-Jeghers syndrome (PJS) is an autosomal-dominant inherited disorder characterized by GI polyps and mucocutaneous-pigmented macules. Peutz-Jeghers syndrome is 1 of the polyposis syndromes, a group of inherited or acquired conditions characterized by the growth of polyps in the GI tract and associated with other extracolonic features. Peutz-Jeghers Syndrome) | STKII | 132 |
| Hereditary pancreatitis Pancreatitis Inflammation of the pancreas. Pancreatitis is classified as acute unless there are computed tomographic or endoscopic retrograde cholangiopancreatographic findings of chronic pancreatitis. The two most common forms of acute pancreatitis are alcoholic pancreatitis and gallstone pancreatitis. Acute Pancreatitis | PRSS1, others | 53 |
| Familial atypical multiple mole Mole Nevi (singular nevus), also known as “moles,” are benign neoplasms of the skin. Nevus is a non-specific medical term because it encompasses both congenital and acquired lesions, hyper- and hypopigmented lesions, and raised or flat lesions. Nevus/Nevi melanoma Melanoma Melanoma is a malignant tumor arising from melanocytes, the melanin-producing cells of the epidermis. These tumors are most common in fair-skinned individuals with a history of excessive sun exposure and sunburns. Melanoma (FAMMM) | p16/CDKN2A | 38 |
| Family history Family History Adult Health Maintenance (increases with the number of 1st-degree relatives and if cancer < 55 years) | Unknown | 32 |
| Lynch syndrome Lynch syndrome Lynch syndrome, also called hereditary non-polyposis colorectal cancer (HNPCC), is the most common inherited colon cancer syndrome, and carries a significantly increased risk for endometrial cancer and other malignancies. Lynch syndrome has an autosomal dominant inheritance pattern involving pathogenic variants in one of the mismatch repair (MMR) genes or epithelial cell adhesion molecule (EpCAM). Lynch syndrome | Mismatched repair genes Genes A category of nucleic acid sequences that function as units of heredity and which code for the basic instructions for the development, reproduction, and maintenance of organisms. DNA Types and Structure | 30 |
| Familial breast/ ovarian cancer Ovarian cancer Ovarian cancer is a malignant tumor arising from the ovarian tissue and is classified according to the type of tissue from which it originates. The 3 major types of ovarian cancer are epithelial ovarian carcinomas (EOCs), ovarian germ cell tumors (OGCTs), and sex cord-stromal tumors (SCSTs). Ovarian Cancer | BRCA2 | 10 |
| Familial breast cancer Familial breast cancer Breast cancers are associated with susceptibility genes. Breast Cancer, others | PALB2 | 6 |
| Ataxia-telangiectasia Ataxia-telangiectasia Ataxia-telangiectasia, also known as Louis-Bar syndrome, is a neurocutaneous syndrome, which involves multiple systems but mainly affects the neurological system. Ataxia-telangiectasia is an autosomal recessive genetic disorder caused by a mutation in the ATM gene (ATM serine/threonine kinase or the ataxia-telangiectasia mutated gene). Ataxia-telangiectasia | ATM | Not yet established |

The pancreas has many functions, served by the endocrine cells in the islets of Langerhans and the exocrine acinar cells. Pancreatic cancer may arise from any of these and disrupt any of their functions.
Image: “2424 Exocrine and Endocrine Pancreas” by OpenStax College. License: CC BY 3.0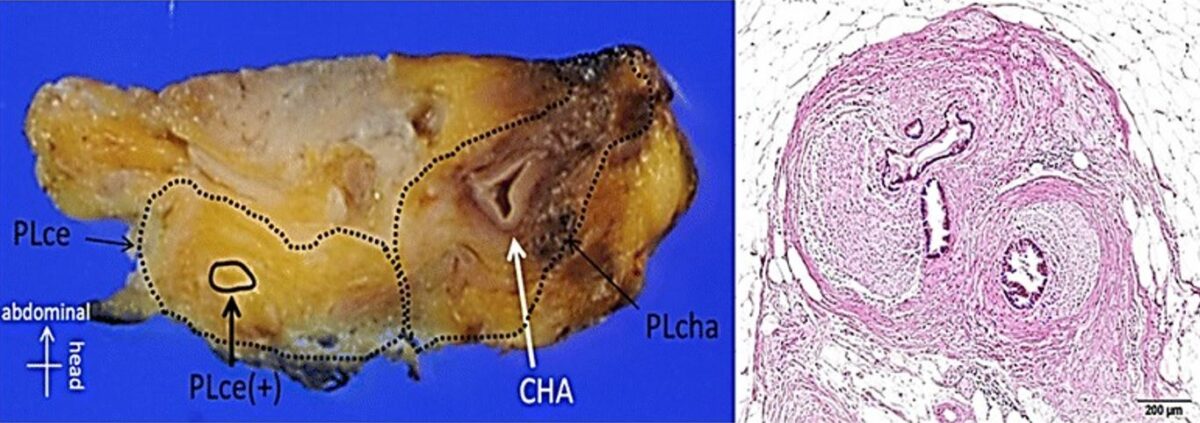
Left image: surgical specimen of an initially unresectable pancreatic carcinoma after chemoradiotherapy
Right image: H&E staining showed the residual cancer cells present at the area encircled with the broken line.
Pancreatic cancer is usually discovered late because of the retroperitoneal Retroperitoneal Peritoneum: Anatomy position of the pancreas Pancreas The pancreas lies mostly posterior to the stomach and extends across the posterior abdominal wall from the duodenum on the right to the spleen on the left. This organ has both exocrine and endocrine tissue. Pancreas: Anatomy.
Imaging and biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma are indicated for patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with suspected PDAC ( pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways, weight loss Weight loss Decrease in existing body weight. Bariatric Surgery, jaundice Jaundice Jaundice is the abnormal yellowing of the skin and/or sclera caused by the accumulation of bilirubin. Hyperbilirubinemia is caused by either an increase in bilirubin production or a decrease in the hepatic uptake, conjugation, or excretion of bilirubin. Jaundice).
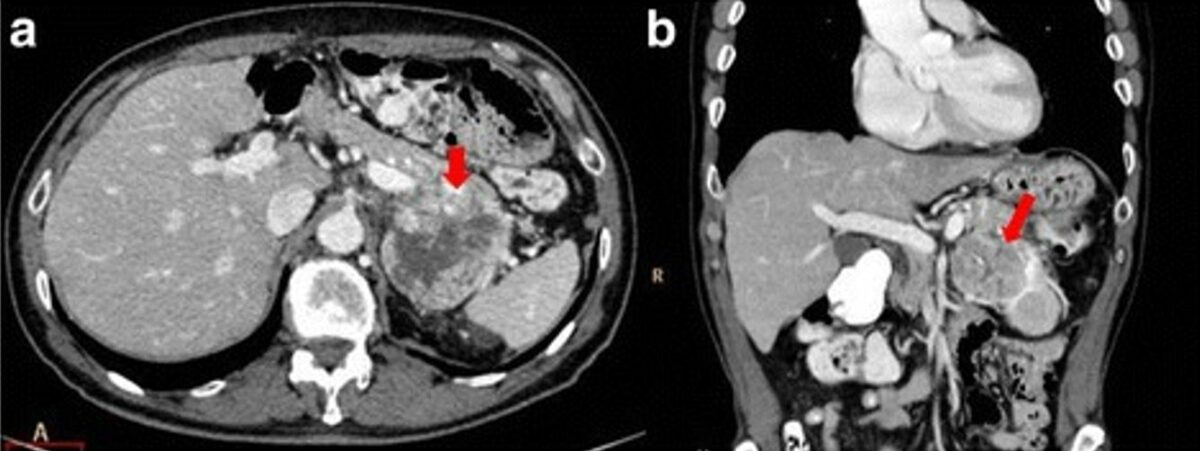
Pancreatic adenocarcinoma of the tail that infiltrates the vascular hilum of the spleen (arrows).
CT scans in the axial plane (a) and coronal plane (b); multiplanar reconstruction (MPR) during the portal phase of dynamic contrast study
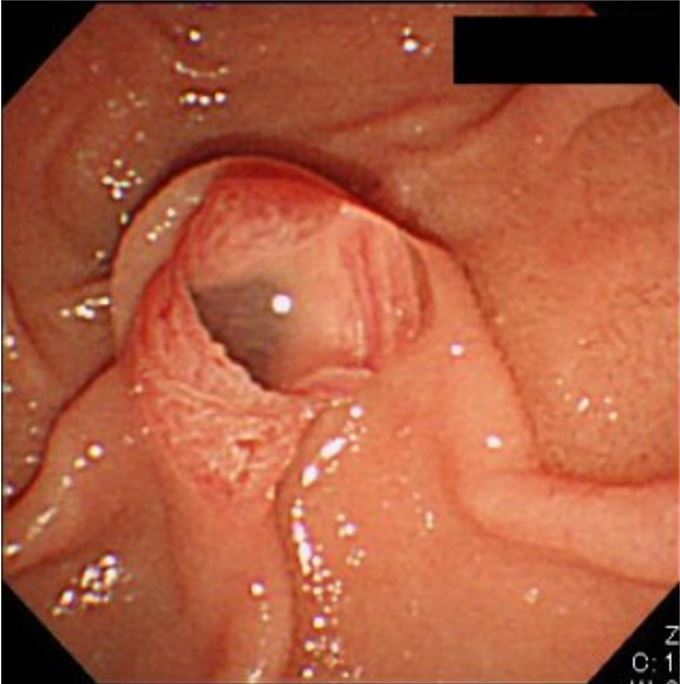
Patulous orifice of a dilated papilla with a protrusion of thick mucus, often seen in patients with intraductal papillary mucinous neoplasm (IPMN), particularly main duct‐type IPMNs, which are one of the precursors to PDAC
Image: “Patulous orifice of a dilated papilla with a protrusion of mucus” by Tanaka, M. License: CC BY 4.0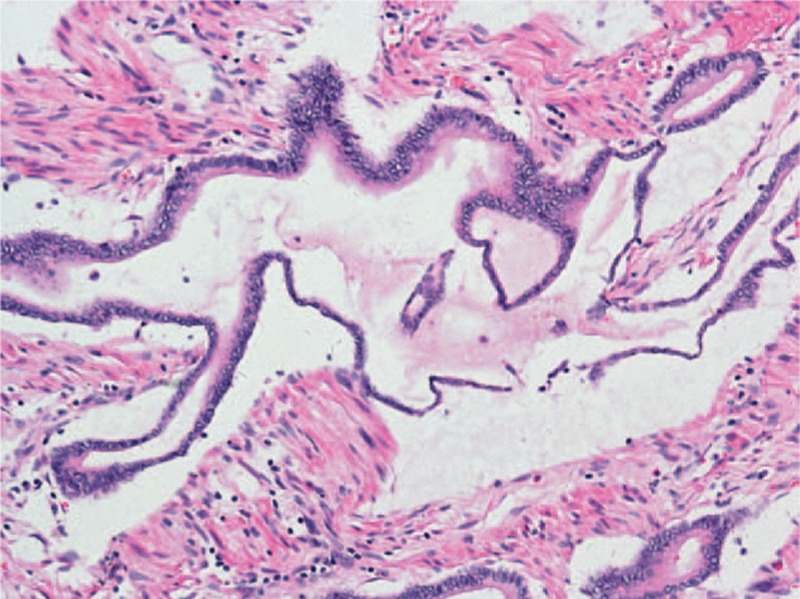
Microphotograph of pancreatic mucinous adenocarcinoma (H&E stain)
Image: “Pathological findings” by Zhang G, Cao Z, Yang G, Wu W, Zhang T, Zhao Y. License: CC BY 4.0Approximately 80%–85% of PDAC tumors are not resectable at the time of presentation.
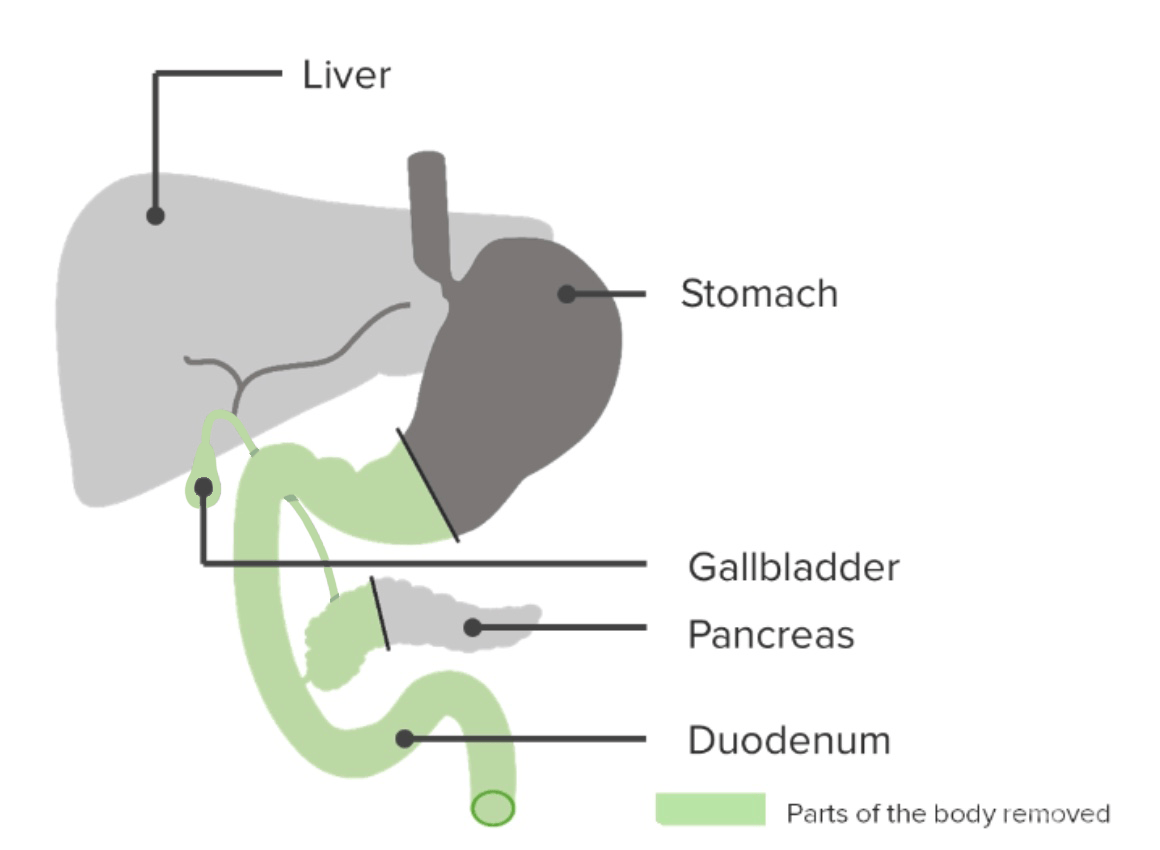
Whipple procedure: pancreaticoduodenectomy
Image by Lecturio. License: CC BY-NC-SA 4.0