Pancreatic neuroendocrine tumors Neuroendocrine tumors Tumors whose cells possess secretory granules and originate from the neuroectoderm, i.e., the cells of the ectoblast or epiblast that program the neuroendocrine system. Common properties across most neuroendocrine tumors include ectopic hormone production (often via apud cells), the presence of tumor-associated antigens, and isozyme composition. Gastrinoma (PanNETs) arise from the endocrine pancreas Endocrine pancreas Pancreas: Anatomy (islet cells) and represent 2%–5% of primary pancreatic neoplasms Neoplasms New abnormal growth of tissue. Malignant neoplasms show a greater degree of anaplasia and have the properties of invasion and metastasis, compared to benign neoplasms. Benign Bone Tumors; the other 95%–98% of pancreatic neoplasms Neoplasms New abnormal growth of tissue. Malignant neoplasms show a greater degree of anaplasia and have the properties of invasion and metastasis, compared to benign neoplasms. Benign Bone Tumors are from the exocrine pancreas Exocrine pancreas The major component (about 80%) of the pancreas composed of acinar functional units of tubular and spherical cells. The acinar cells synthesize and secrete several digestive enzymes such as trypsinogen; lipase; amylase; and ribonuclease. Secretion from the exocrine pancreas drains into the pancreatic ductal system and empties into the duodenum. Pancreas: Anatomy. The majority of PanNETs are nonfunctional (50%–75%), while others that are functional may be benign Benign Fibroadenoma or malignant. Benign Benign Fibroadenoma or malignant PanNETs include insulinomas Insulinomas An insulinoma is a type of functional neuroendocrine tumor that manifests with hypoglycemia due to autologous secretion of insulin. It more commonly presents as a solitary benign tumor but can sometimes be associated with MEN type 1 (MEN1). Patients present with fasting hypoglycemia, which may manifest as episodes of diaphoresis, palpitations, tremor, and confusion. Insulinomas, gastrinomas, malignant pancreatic neuroendocrine carcinomas, and very rare tumors named glucagonomas, somatostatinomas, and VIPomas, after the hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types they secrete. Diagnosis is made clinically, with lab testing for hormone secretion Secretion Coagulation Studies, as well as imaging or upper endoscopy Endoscopy Procedures of applying endoscopes for disease diagnosis and treatment. Endoscopy involves passing an optical instrument through a small incision in the skin i.e., percutaneous; or through a natural orifice and along natural body pathways such as the digestive tract; and/or through an incision in the wall of a tubular structure or organ, i.e. Transluminal, to examine or perform surgery on the interior parts of the body. Gastroesophageal Reflux Disease (GERD). Management is surgical or with newer molecularly targeted therapies.
Last updated: Jan 31, 2023
3 of the most common somatic mutations:
Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship may present with metastatic disease in the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy, which is then confirmed with biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma. The diagnostic approach focuses on identifying both the extent of disease spread and the likely primary site of disease.
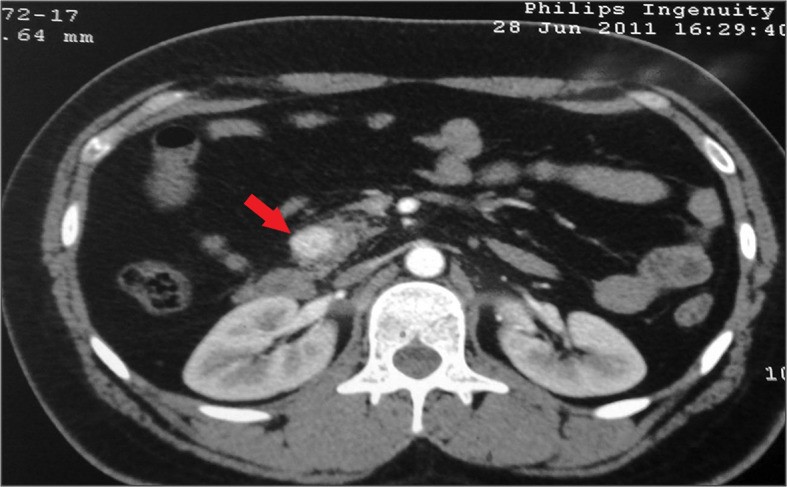
Contrast-enhanced CT (transverse section) showing a solitary insulinoma in the head of the pancreas (arrow)
Image: “Insulinoma presenting as refractory seizure disorder” by Correia P, Panchani R, Ranjan R, Agrawal C. License: CC BY 3.0, edited by Lecturio.In general, management is surgical if the neoplasm is resectable.