Drug-induced liver injury (DILI) is the most common cause of acute liver failure (ALF). Hepatotoxic drugs can cause dose-dependent, direct liver injury or idiosyncratic reactions mediated by immune or metabolic processes. This injury can result in hepatitis, cholestasis, steatosis, or overlapping changes. The presentation can be acute or chronic, with severe toxicity manifesting as fulminant liver failure. The diagnosis of DILI requires a thorough history, liver function tests, and drug levels, if available. Management consists of discontinuing the drug, supportive therapy, and monitoring for complications. Acetaminophen is the most common cause of DILI and is treated with a specific therapy, N-acetylcysteine (NAC).
Last updated: Jan 17, 2024
The liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy handles metabolism of drugs/toxins, thus making the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy susceptible to injury. The drugs themselves undergo processes to be inactivated and become water soluble (for proper renal or biliary excretion).
Phase I reaction:
Phase II reaction:
Phase III reaction:
Intrinsic/direct hepatotoxins:
Idiosyncratic reactions:
Potential mechanism overlaps of drugs may occur as mixed hepatocellular and cholestatic changes occur.
Potential mechanisms of how drugs cause liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy cell injury Cell injury The cell undergoes a variety of changes in response to injury, which may or may not lead to cell death. Injurious stimuli trigger the process of cellular adaptation, whereby cells respond to withstand the harmful changes in their environment. Overwhelmed adaptive mechanisms lead to cell injury. Mild stimuli produce reversible injury. If the stimulus is severe or persistent, injury becomes irreversible. Cell Injury and Death:
Potential mechanisms of how drugs affect the biliary excretion pathway:
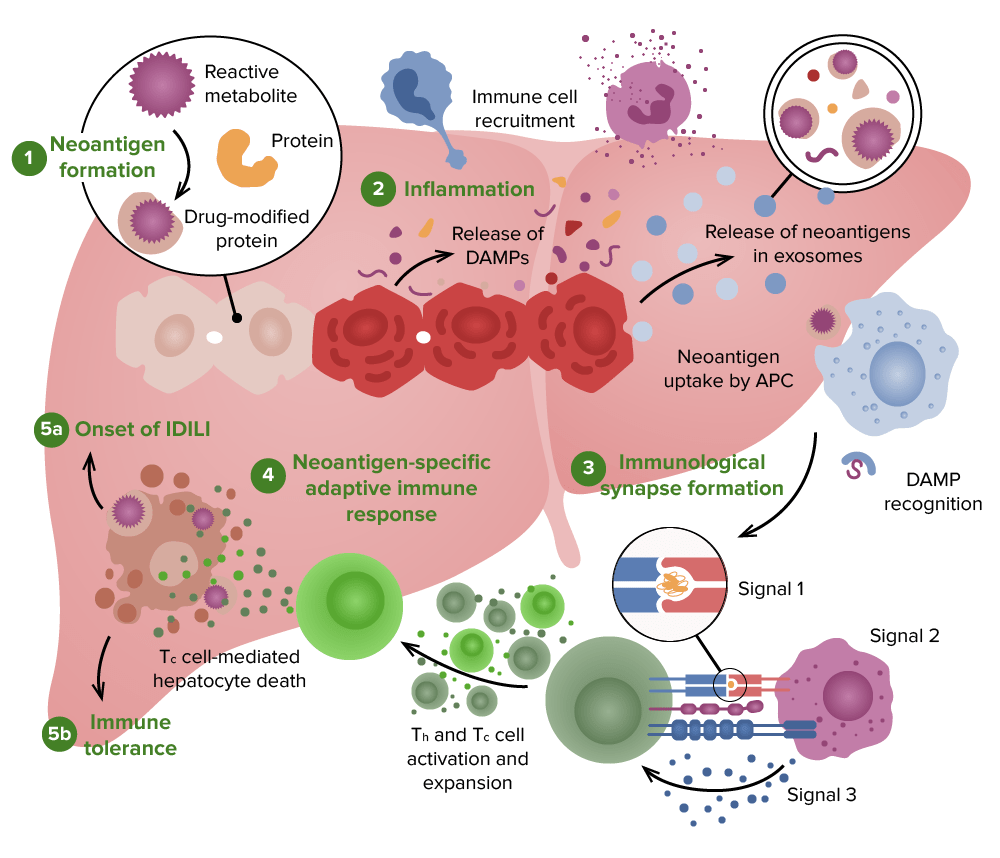
Possible pathophysiology of drug-induced liver injury:
1. A reactive metabolite may be formed by hepatocytes, which can covalently bond to proteins to form neoantigens.
2. A release of neoantigens and damage-associated molecular pattern (DAMP) molecules from damaged hepatocytes, which can lead to activation of antigen-presenting cells (APCs) and recruitment of innate immune cells.
3. APC activation leads to the expression of signal 1 and signal 2
4. T helper (Th) cells and cytotoxic T (Tc) cells are activated, leading to an adaptive immune response.
5. 5a: The dominant adaptive immune response in idiosyncratic drug-induced liver injury (IDILI) is usually a cell-mediated immune response.
5b: However, if the binding of the drug or drug-modified peptide is not very strong, the adaptive immune response will end in immune tolerance, preventing or limiting liver injury.
Acute liver failure Liver failure Severe inability of the liver to perform its normal metabolic functions, as evidenced by severe jaundice and abnormal serum levels of ammonia; bilirubin; alkaline phosphatase; aspartate aminotransferase; lactate dehydrogenases; and albumin/globulin ratio. Autoimmune Hepatitis:
Chronic liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy injury:
Chronic liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy injury is defined as lasting > 3 months, thereby resembling chronic liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy disease or cirrhosis Cirrhosis Cirrhosis is a late stage of hepatic parenchymal necrosis and scarring (fibrosis) most commonly due to hepatitis C infection and alcoholic liver disease. Patients may present with jaundice, ascites, and hepatosplenomegaly. Cirrhosis can also cause complications such as hepatic encephalopathy, portal hypertension, portal vein thrombosis, and hepatorenal syndrome. Cirrhosis:
Liver function tests Liver function tests Liver function tests, also known as hepatic function panels, are one of the most commonly performed screening blood tests. Such tests are also used to detect, evaluate, and monitor acute and chronic liver diseases. Liver Function Tests:
Drug levels:
| Type of injury | Blood test |
|---|---|
| Hepatitis |
|
| Cholestasis |
|
| Mixed |
|
Histologic patterns of injury:
| Pattern of injury | Examples |
|---|---|
| Acute hepatitis Acute Hepatitis Autoimmune Hepatitis |
|
| Chronic hepatitis/ fibrosis Fibrosis Any pathological condition where fibrous connective tissue invades any organ, usually as a consequence of inflammation or other injury. Bronchiolitis Obliterans |
|
| Cholestatic hepatitis (mixed) |
|
| Cholestasis |
|
| Steatosis Steatosis Nonalcoholic Fatty Liver Disease or steatohepatitis |
|
| Granulomas Granulomas A relatively small nodular inflammatory lesion containing grouped mononuclear phagocytes, caused by infectious and noninfectious agents. Sarcoidosis |
|
| Vascular lesions |
|
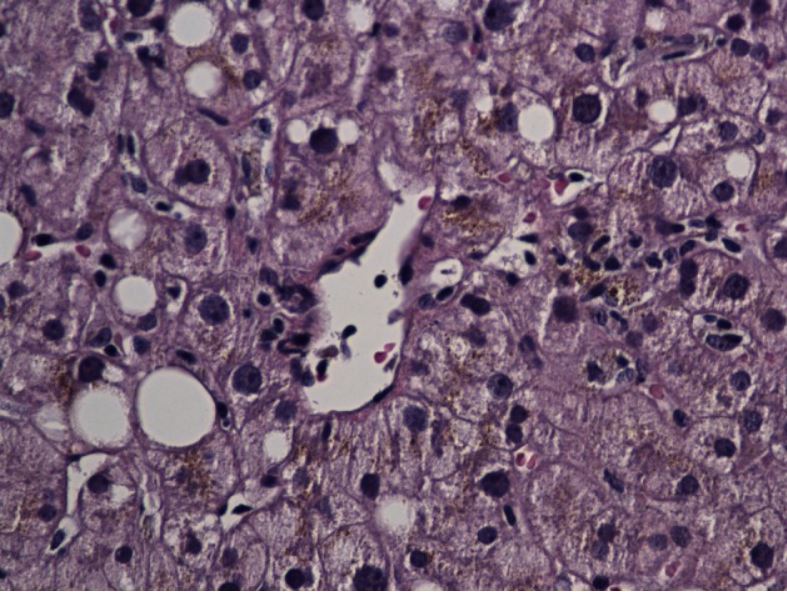
Liver biopsy specimen showing lobular hepatitis secondary to ibuprofen
Image: “A rare coexistence: drug-induced hepatitis and meningitis in association with Ibuprofen” by Nayudu SK, Kavuturu S, Niazi M, Daniel M, Dev A, Kumbum K. License: CC BY 2.0.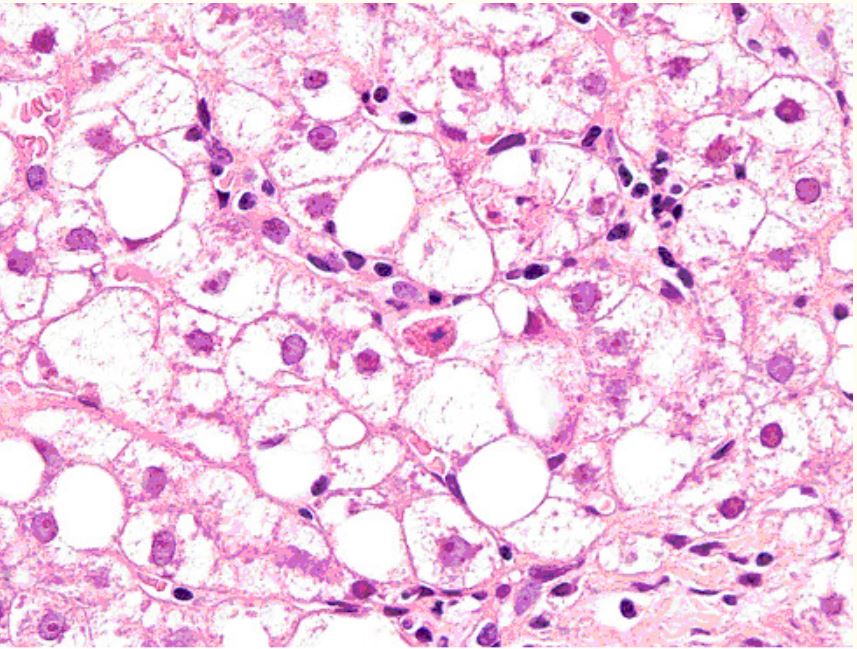
Liver biopsy of a patient with human immunodeficiency virus (HIV) infection taking stavudine
Laboratory tests showed elevated transaminases; image showed acidophil bodies, foamy degeneration of hepatocytes (steatosis).
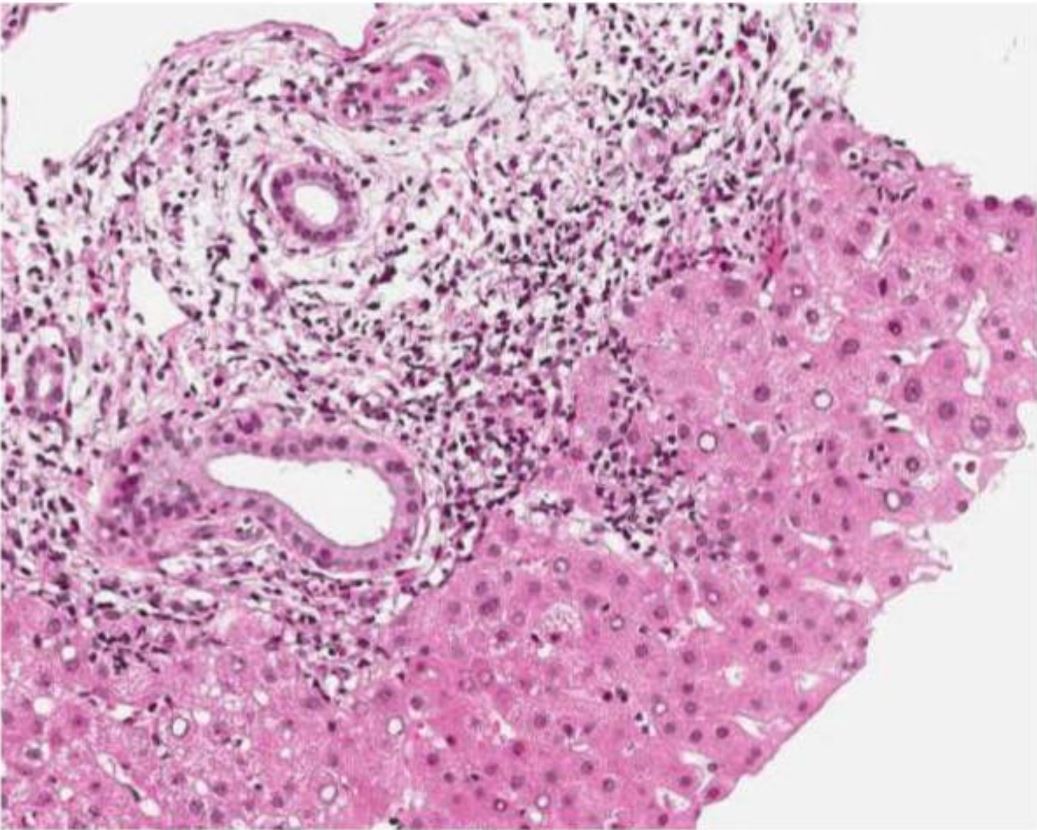
Liver biopsy of a patient with elevated liver enzymes after treatment of Helicobacter pylori infection (using clarithromycin and amoxicillin):
Image shows portal inflammation.
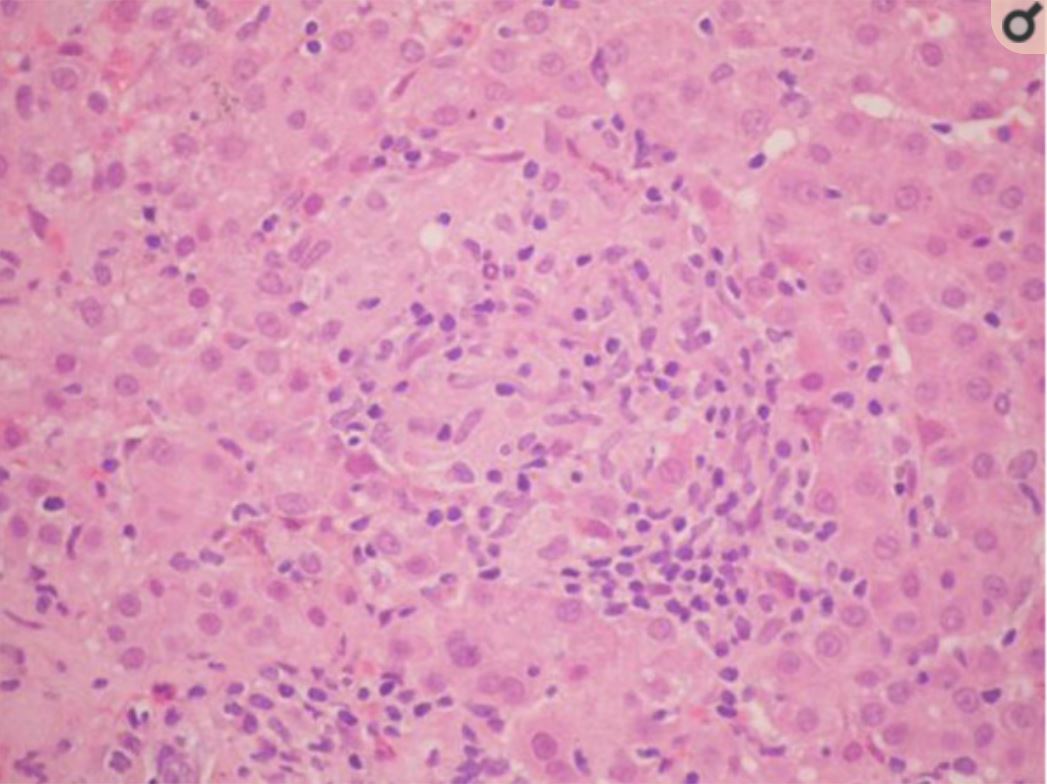
Image of albendazole-induced granulomatous hepatitis:
hepatic parenchyma with epithelioid macrophages that have formed granulomas without necrosis, interspersed with lymphocytes
Limited specific treatments for DILI:
Pathogenesis:
Clinical presentation:
Diagnosis:
Initial management:
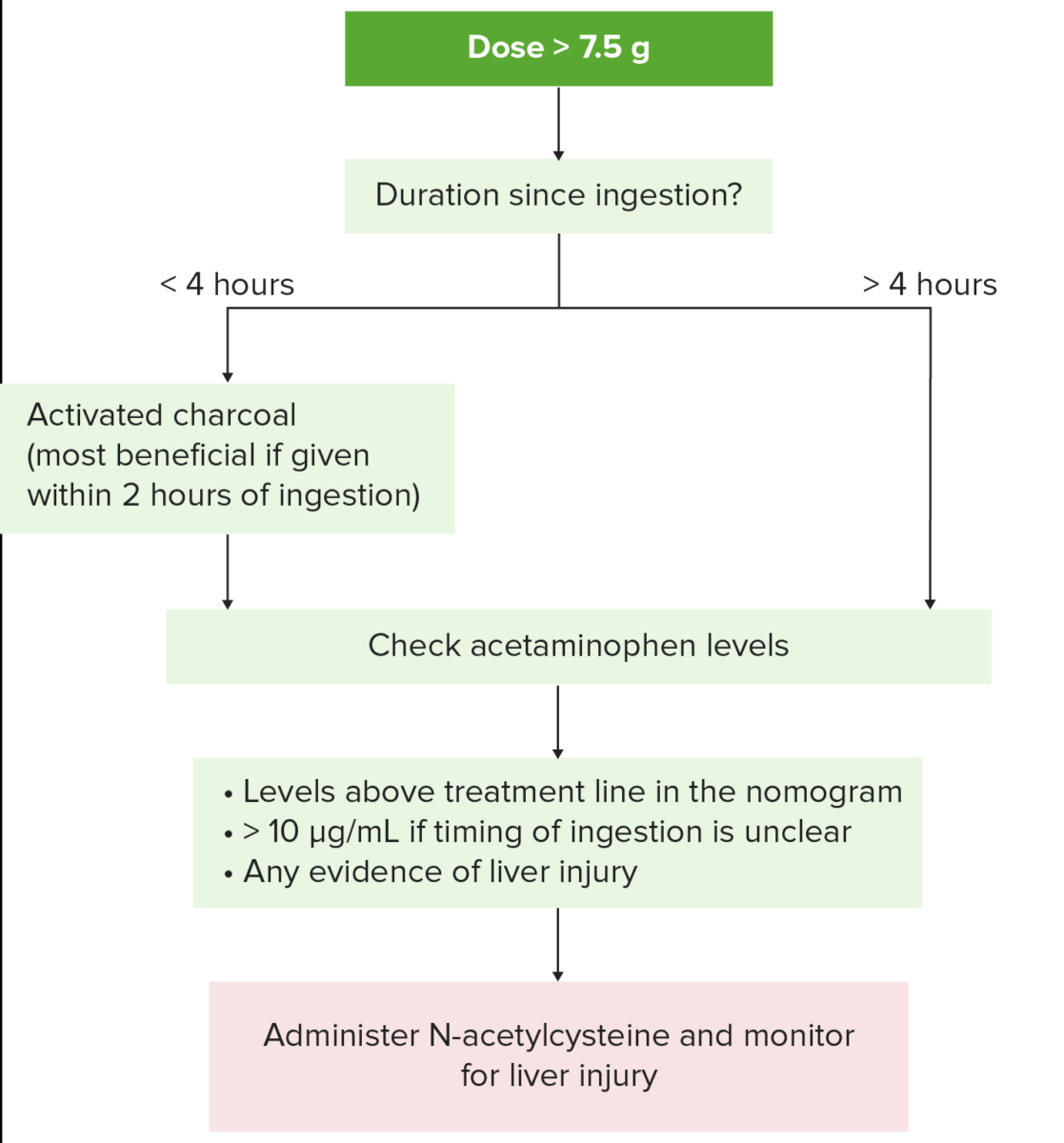
Management of acetaminophen toxicity:
1. Obtain history, identify agents involved, and determine severity and possible drug toxicity.
If acetaminophen ingestion (potentially toxic dose > 7.5 g) has been < 4 hours, activated charcoal is given to prevent absorption of residual drug.
Patient should be alert to protect the airway and avoid aspiration.
2. Serum acetaminophen level is obtained (recommended at 4 hours after ingestion; 2nd drug level is obtained later if extended release preparation was ingested).
3. N-acetyl-cysteine is administered in the following cases:
Levels above the treatment line in the nomogram
Unclear time of ingestion and serum acetaminophen level is > 10 µg/mL
Evidence of hepatotoxicity
Suspected single dose of > 7.5 g or 150 mg/kg and result of acetaminophen level will not be available for at least 8 hours.
Management:
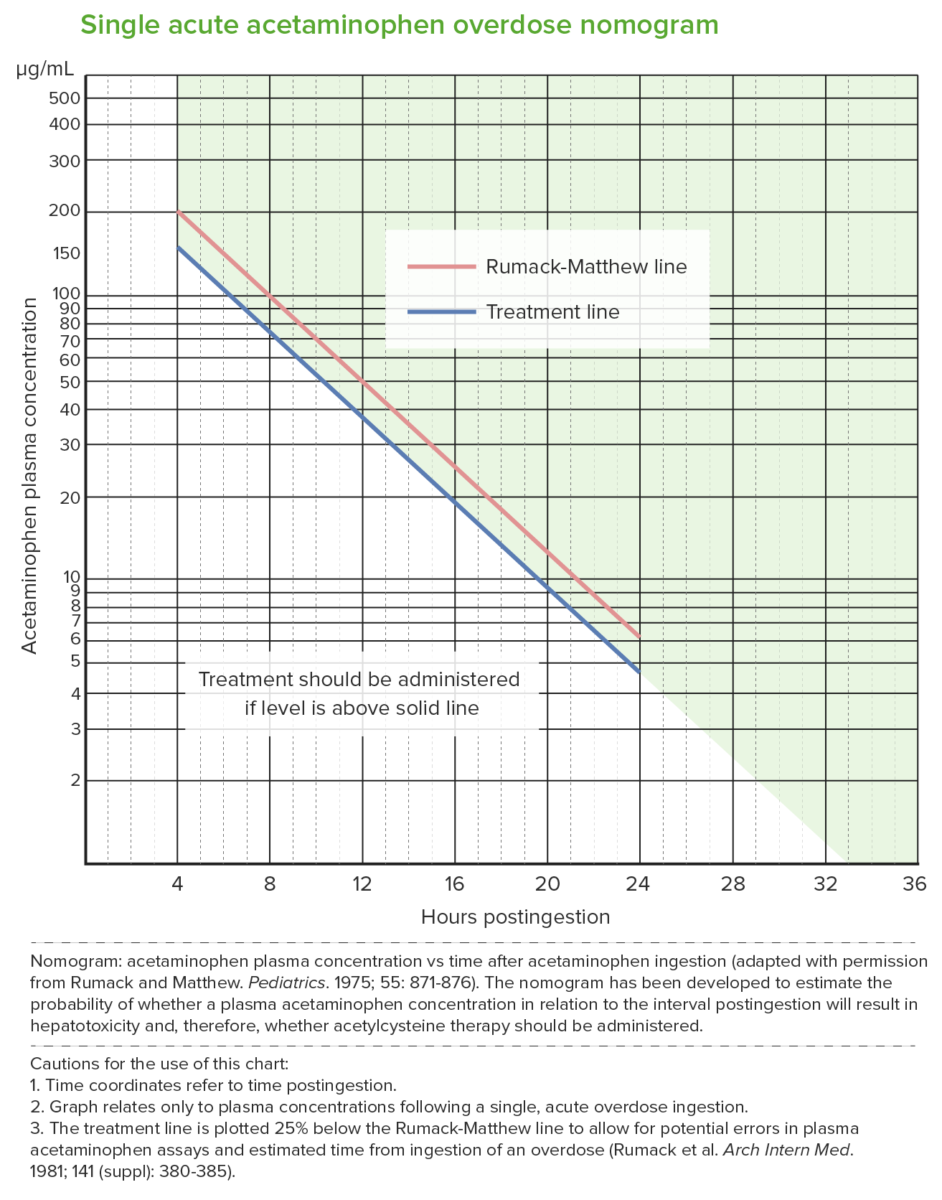
Rumack-Matthew nomogram (acetaminophen toxicity nomogram):
Used after a single acute acetaminophen ingestion.
It predicts potential hepatotoxicity beginning at 4 hours after ingestion. Levels measured earlier than 4 hours may not be reliable.
The nomogram cannot be used for ingestions that occurred > 24 hours prior to presentation.
The upper (red) line is the Rumack-Matthew line; values above this line develop toxicity (noted in 60%).
The lower (blue) line is the treatment line (U.S. Food and Drug Administration required treatment line to be 25% below the original line).
NAC treatment is given when the acetaminophen level is at the treatment line 4 hours post ingestion (which is below the toxicity threshold).