Acetaminophen Acetaminophen Acetaminophen is an over-the-counter nonopioid analgesic and antipyretic medication and the most commonly used analgesic worldwide. Despite the widespread use of acetaminophen, its mechanism of action is not entirely understood. Acetaminophen (APAP) is an over-the-counter nonopioid analgesic Nonopioid Analgesic Acetaminophen and antipyretic Antipyretic Acetaminophen medication. Acetaminophen Acetaminophen Acetaminophen is an over-the-counter nonopioid analgesic and antipyretic medication and the most commonly used analgesic worldwide. Despite the widespread use of acetaminophen, its mechanism of action is not entirely understood. Acetaminophen is the most commonly used analgesic worldwide. Acetaminophen Acetaminophen Acetaminophen is an over-the-counter nonopioid analgesic and antipyretic medication and the most commonly used analgesic worldwide. Despite the widespread use of acetaminophen, its mechanism of action is not entirely understood. Acetaminophen overdose is also one of the most common causes of medication poisoning and death. Because APAP is primarily metabolized by the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy, overdose can lead to life threatening hepatotoxicity Hepatotoxicity Acetaminophen. In adults, limitation of total APAP dose (from all sources and routes) to < 4,000 mg per day is recommended. During evaluation, a thorough history and measurement of serum APAP are important, as the initial clinical signs of APAP overdose can be nonspecific. Management of overdose includes drug serum concentration, stabilization, decontamination, and administration of N-acetylcysteine (NAC). Use of NAC within 8 hours of ingestion is associated with good case outcomes. Without treatment, however, cases are at significant risk of severe hepatotoxicity Hepatotoxicity Acetaminophen and potentially death.
Last updated: May 14, 2025
The clinical course of APAP poisoning is often divided into 4 stages that are classified according to duration since time of ingestion.
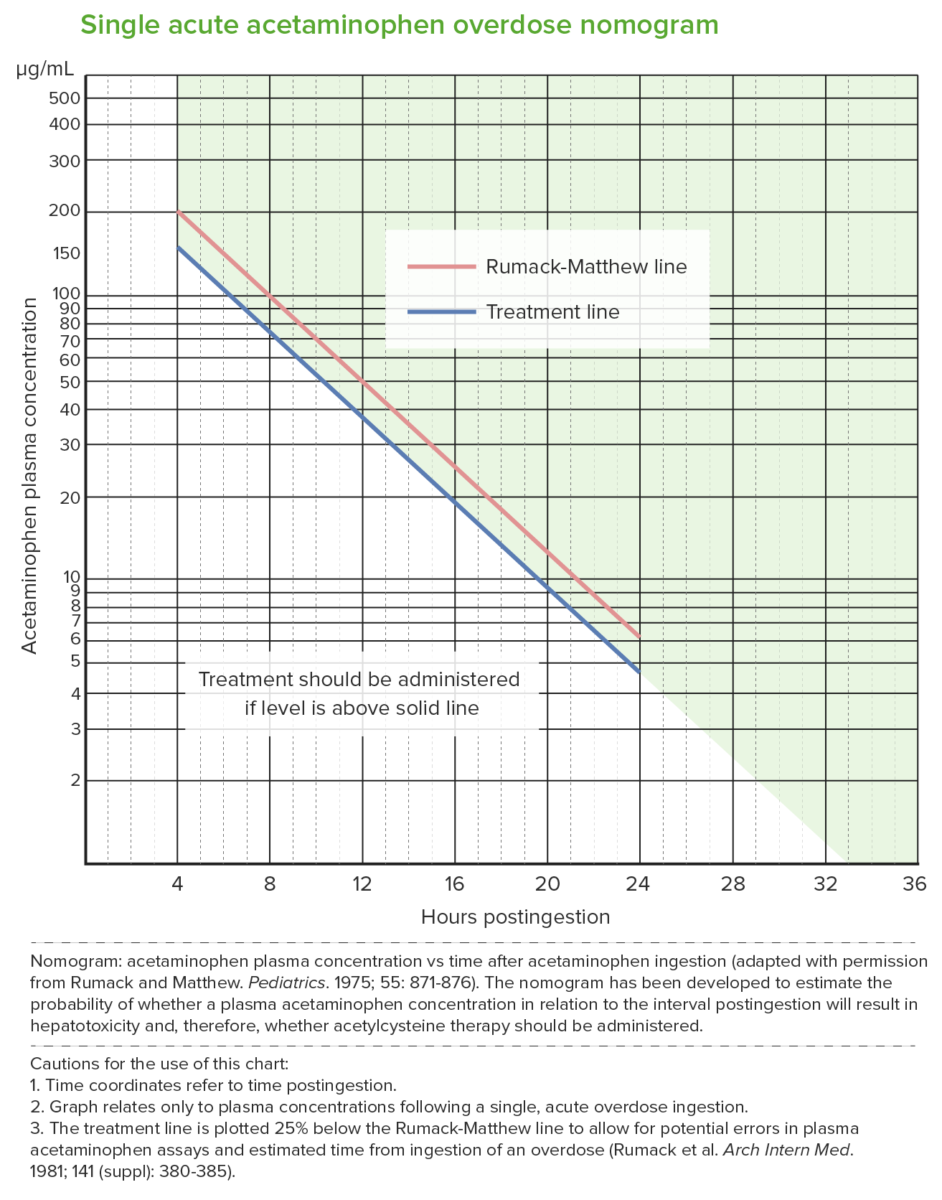
Rumack-Matthew nomogram (acetaminophen toxicity nomogram):
Used after a single acute acetaminophen ingestion.
The nomogram predicts potential hepatotoxicity beginning at 4 hours after ingestion. Levels measured earlier than 4 hours may not be reliable.
The nomogram cannot be used for ingestions that occurred > 24 hours prior to presentation.
The upper (red) line is the Rumack-Matthew line; values above this line develop toxicity (noted in 60%).
The lower (blue) line is the treatment line (U.S. Food and Drug Administration required treatment line to be 25% below the original line).
NAC treatment is given when the acetaminophen level is at the treatment line 4 hours post ingestion (which is below the toxicity threshold).
Entails supportive care, limiting drug absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption, and treatment of toxic effects:
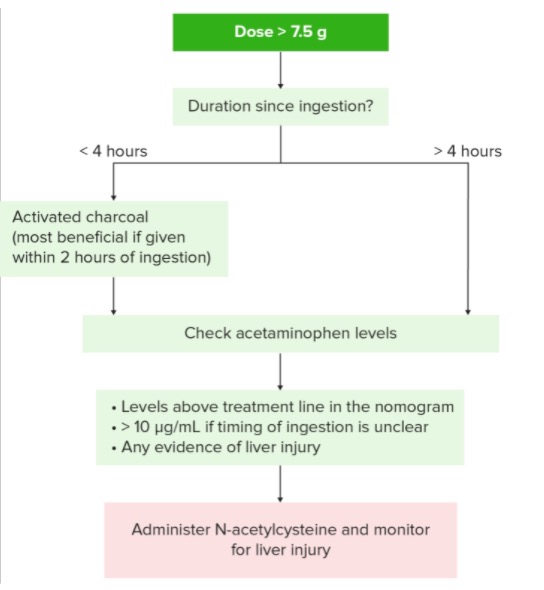
Overview of the treatment algorithm for acetaminophen toxicity
Image by Lecturio.