Thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy cancer is a malignancy Malignancy Hemothorax arising from the thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy gland cells: thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy follicular cells (papillary, follicular, and anaplastic carcinomas) and calcitonin-producing C cells C cells Calcium Hemostasis and Bone Metabolism (medullary carcinomas). Rare cancers are derived from the lymphocytes Lymphocytes Lymphocytes are heterogeneous WBCs involved in immune response. Lymphocytes develop from the bone marrow, starting from hematopoietic stem cells (HSCs) and progressing to common lymphoid progenitors (CLPs). B and T lymphocytes and natural killer (NK) cells arise from the lineage. Lymphocytes: Histology ( lymphoma Lymphoma A general term for various neoplastic diseases of the lymphoid tissue. Imaging of the Mediastinum) and/or stromal and vascular elements (sarcoma). Driver mutations involving the receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors tyrosine Tyrosine A non-essential amino acid. In animals it is synthesized from phenylalanine. It is also the precursor of epinephrine; thyroid hormones; and melanin. Synthesis of Nonessential Amino Acids kinase pathway (such as RET and BRAF) and a family history Family History Adult Health Maintenance of cancer or related syndromes increase the risk. Exposure to ionizing radiation Radiation Emission or propagation of acoustic waves (sound), electromagnetic energy waves (such as light; radio waves; gamma rays; or x-rays), or a stream of subatomic particles (such as electrons; neutrons; protons; or alpha particles). Osteosarcoma and iodine Iodine A nonmetallic element of the halogen group that is represented by the atomic symbol I, atomic number 53, and atomic weight of 126. 90. It is a nutritionally essential element, especially important in thyroid hormone synthesis. In solution, it has anti-infective properties and is used topically. Thyroid Hormones deficiency are also considered risk factors. The major types can present as thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy nodules or enlarged cervical lymph nodes Lymph Nodes They are oval or bean shaped bodies (1 - 30 mm in diameter) located along the lymphatic system. Lymphatic Drainage System: Anatomy. The diagnostic approach includes thyroid-stimulating hormone Thyroid-stimulating hormone A glycoprotein hormone secreted by the adenohypophysis. Thyrotropin stimulates thyroid gland by increasing the iodide transport, synthesis and release of thyroid hormones (thyroxine and triiodothyronine). Thyroid Hormones, ultrasonography, and biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma. Treatment options are surgical removal of the thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy gland, with the addition of radioactive iodine Radioactive iodine Unstable isotopes of iodine that decay or disintegrate emitting radiation. I atoms with atomic weights 117-139, except I 127, are radioactive iodine isotopes. Antithyroid Drugs therapy and systemic therapy, depending on the type and extent of the thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy malignancy Malignancy Hemothorax.
Last updated: May 17, 2024
Thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy cancer is a malignancy Malignancy Hemothorax arising from the cells of the thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy gland, including thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy follicular cells (thyrocytes), calcitonin-producing C cells C cells Calcium Hemostasis and Bone Metabolism, lymphocytes Lymphocytes Lymphocytes are heterogeneous WBCs involved in immune response. Lymphocytes develop from the bone marrow, starting from hematopoietic stem cells (HSCs) and progressing to common lymphoid progenitors (CLPs). B and T lymphocytes and natural killer (NK) cells arise from the lineage. Lymphocytes: Histology, and/or stromal and vascular elements.
Thyroid cancer:
Malignancy presents as a solitary thyroid nodule.
| Type | Incidence Incidence The number of new cases of a given disease during a given period in a specified population. It also is used for the rate at which new events occur in a defined population. It is differentiated from prevalence, which refers to all cases in the population at a given time. Measures of Disease Frequency | Clinical features |
|---|---|---|
| Differentiated (originating from thyrocytes) | ||
| Papillary carcinoma |
|
|
| Follicular carcinoma |
|
|
| Undifferentiated (originating from thyrocytes) | ||
| Anaplastic carcinoma |
|
|
| Originating from parafollicular C cells C cells Calcium Hemostasis and Bone Metabolism | ||
| Medullary carcinoma |
|
|
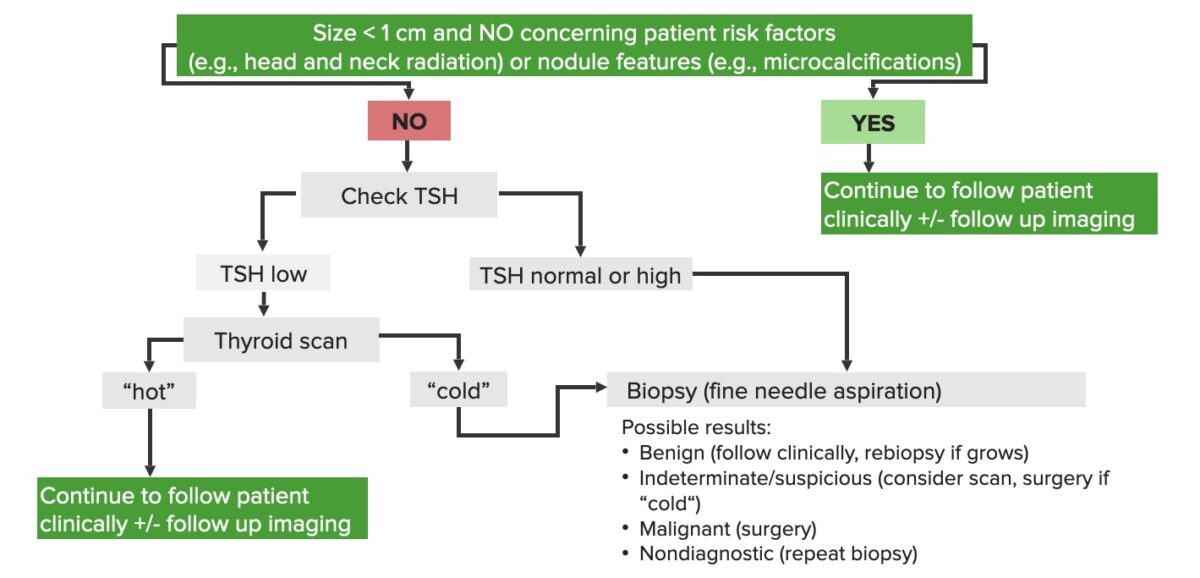
Schematic diagram of the diagnostic approach to thyroid nodules
Image by Lecturio.| Type | Histopathologic features |
|---|---|
| Papillary carcinoma |
|
| Follicular carcinoma |
|
| Medullary carcinoma |
|
| Anaplastic carcinoma |
|
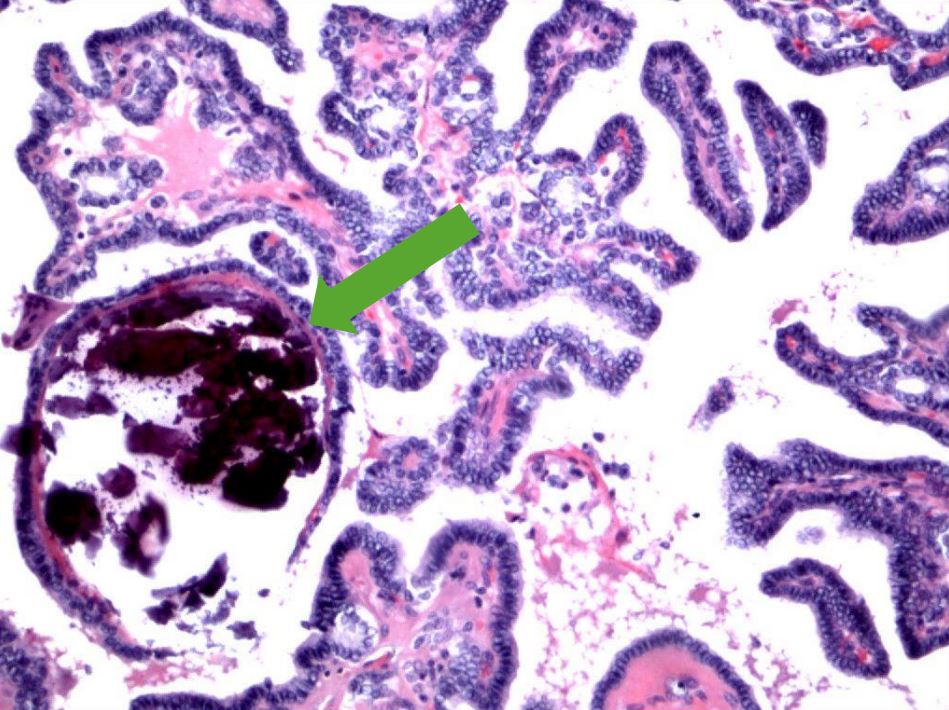
Papillary thyroid carcinoma:
Note the branching papillae and blue concretions representing psammoma bodies (arrow).
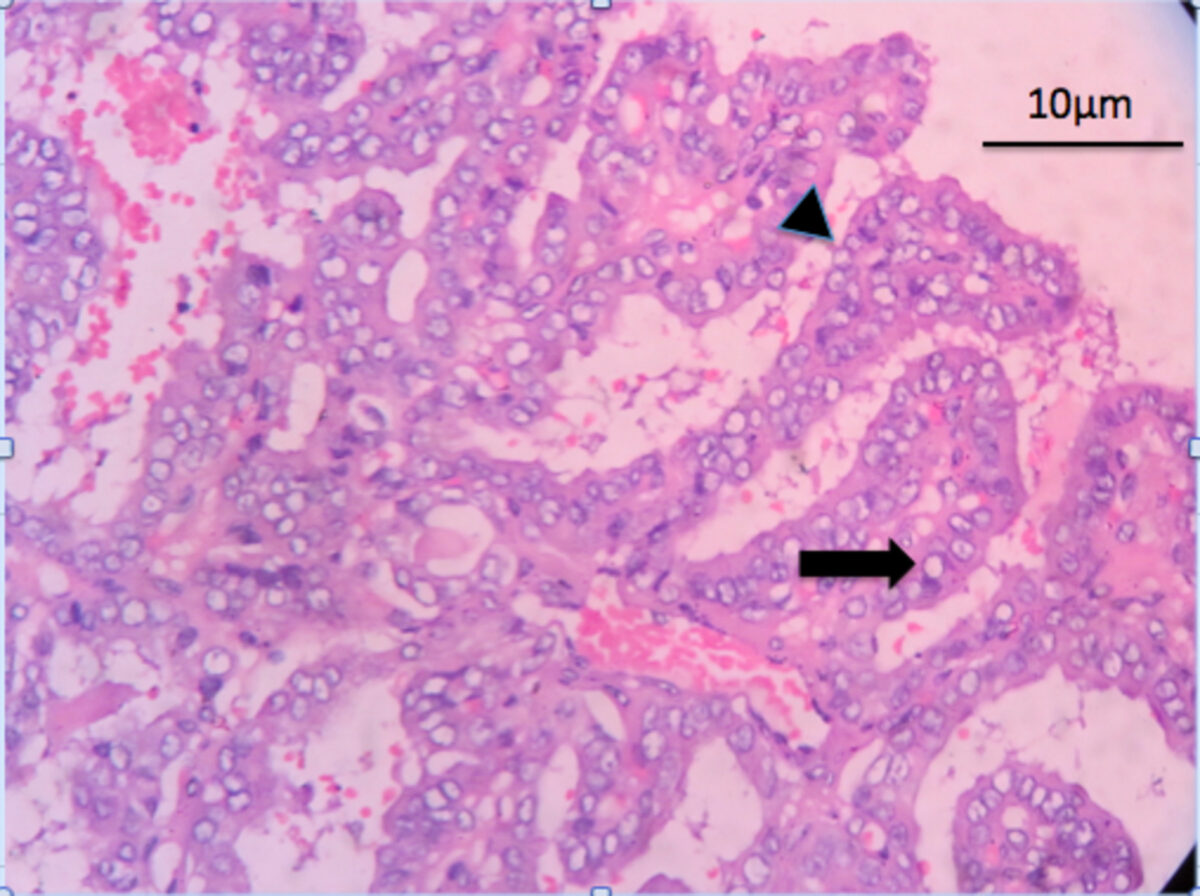
Features of papillary thyroid carcinoma:
H&E staining showing complex papillary architecture (arrowhead) with a fibrovascular core. The tumor cells have nuclear clearing: Orphan Annie eye nuclei (arrow)
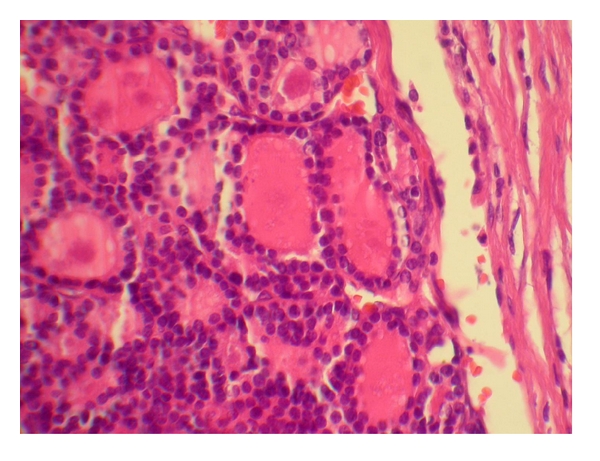
Follicular thyroid carcinoma: carcinoma forming follicles with uniform small nuclei
Image: “Occult follicular thyroid carcinoma presenting as a frontal bone metastasis: a case report” by Tahamtan M, Mokhtari M, Pakbaz S, Tahamtan M. License: CC BY 3.0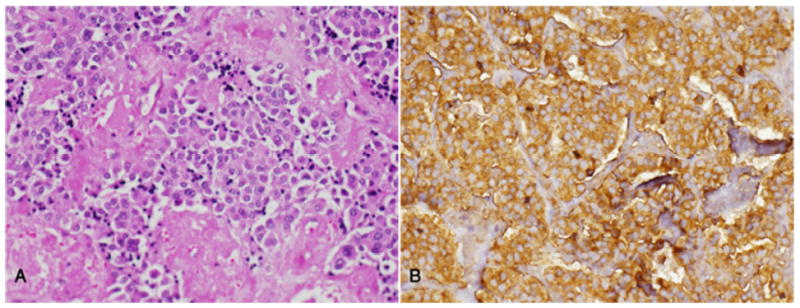
Medullary thyroid carcinoma:
A. Note the sheets and nests of tumor cells in an amyloid stroma.
B. Strong immunopositivity for calcitonin in all tumor cells
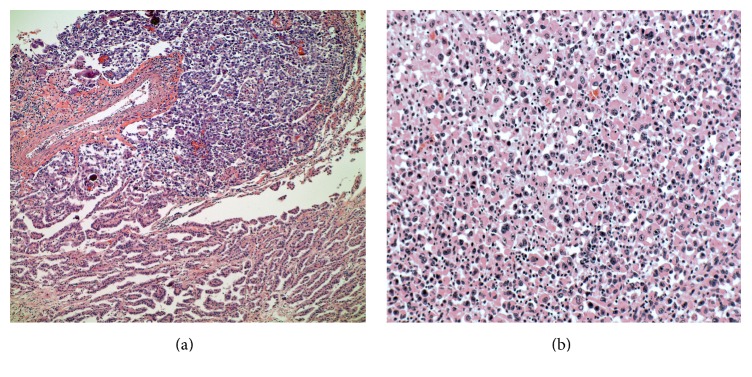
Anaplastic thyroid carcinoma (papillary carcinoma transforming into anaplastic type):
(a) 10×: Papillary thyroid carcinoma (lower) with transformation to anaplastic carcinoma (upper)
(b) 20×: Higher-power view of the anaplastic carcinoma component with enlarged nuclei, prominent nucleoli, and moderate amounts of eosinophilic cytoplasm
Further tests should be obtained after diagnosis: