Celiac disease (also known as celiac sprue or gluten enteropathy Enteropathy IPEX Syndrome) is an autoimmune reaction to gliadin, which is a component of gluten. Celiac disease is closely associated with HLA-DQ2 and HLA-DQ8. The immune response is localized to the proximal small intestine Small intestine The small intestine is the longest part of the GI tract, extending from the pyloric orifice of the stomach to the ileocecal junction. The small intestine is the major organ responsible for chemical digestion and absorption of nutrients. It is divided into 3 segments: the duodenum, the jejunum, and the ileum. Small Intestine: Anatomy and causes the characteristic histologic findings of villous atrophy Villous Atrophy Giardia/Giardiasis, crypt hyperplasia Hyperplasia An increase in the number of cells in a tissue or organ without tumor formation. It differs from hypertrophy, which is an increase in bulk without an increase in the number of cells. Cellular Adaptation, and intraepithelial lymphocytosis Lymphocytosis WBCs develop from stem cells in the bone marrow and are called leukocytes when circulating in the bloodstream. Lymphocytes are 1 of the 5 subclasses of WBCs. Lymphocytosis is an increase in the number or proportion of the lymphocyte subclass of WBCs, often as a result of an immune response to infection (known as reactive lymphocytosis). Lymphocytosis. Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship typically present with diarrhea Diarrhea Diarrhea is defined as ≥ 3 watery or loose stools in a 24-hour period. There are a multitude of etiologies, which can be classified based on the underlying mechanism of disease. The duration of symptoms (acute or chronic) and characteristics of the stools (e.g., watery, bloody, steatorrheic, mucoid) can help guide further diagnostic evaluation. Diarrhea and symptoms related to malabsorption Malabsorption General term for a group of malnutrition syndromes caused by failure of normal intestinal absorption of nutrients. Malabsorption and Maldigestion ( steatorrhea Steatorrhea A condition that is characterized by chronic fatty diarrhea, a result of abnormal digestion and/or intestinal absorption of fats. Diarrhea, weight loss Weight loss Decrease in existing body weight. Bariatric Surgery, and nutritional deficiencies). Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship are screened with serological antibody testing, and diagnosis is confirmed by small intestine Small intestine The small intestine is the longest part of the GI tract, extending from the pyloric orifice of the stomach to the ileocecal junction. The small intestine is the major organ responsible for chemical digestion and absorption of nutrients. It is divided into 3 segments: the duodenum, the jejunum, and the ileum. Small Intestine: Anatomy biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma. Treatment requires a lifelong gluten-free diet.
Last updated: Dec 15, 2025
Environmental, immunologic, and genetic factors contribute to the disease process:
Gluten peptides trigger Trigger The type of signal that initiates the inspiratory phase by the ventilator Invasive Mechanical Ventilation the innate immune response Innate Immune Response Immunity to pathogens is divided into innate and adaptive immune responses. The innate immune response is the 1st line of defense against a variety of pathogens, including bacteria, fungi, viruses, and parasites. In essentially the same form, the innate type of immunity is present in all multicellular organisms. Innate Immunity: Barriers, Complement, and Cytokines in intestinal epithelial cells, leading to T cell–mediated mucosal damage of the proximal small intestine Small intestine The small intestine is the longest part of the GI tract, extending from the pyloric orifice of the stomach to the ileocecal junction. The small intestine is the major organ responsible for chemical digestion and absorption of nutrients. It is divided into 3 segments: the duodenum, the jejunum, and the ileum. Small Intestine: Anatomy (distal duodenum Duodenum The shortest and widest portion of the small intestine adjacent to the pylorus of the stomach. It is named for having the length equal to about the width of 12 fingers. Small Intestine: Anatomy and proximal jejunum Jejunum The middle portion of the small intestine, between duodenum and ileum. It represents about 2/5 of the remaining portion of the small intestine below duodenum. Small Intestine: Anatomy).
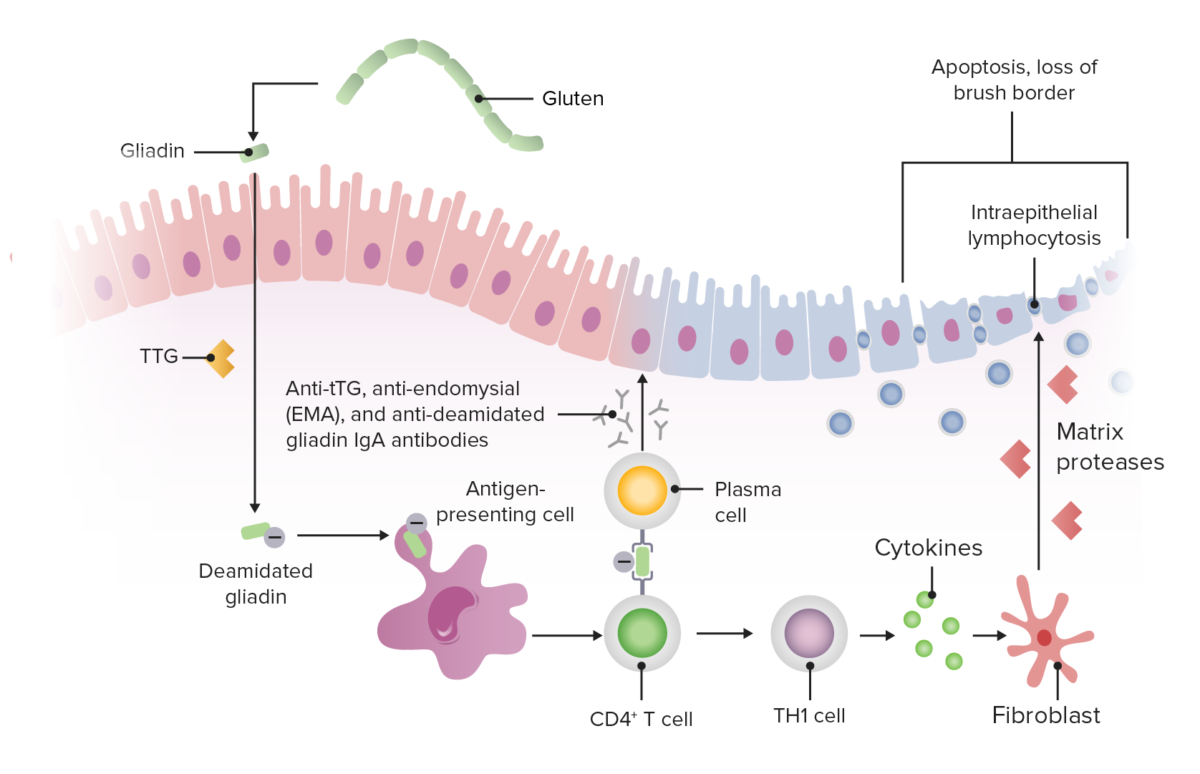
Pathophysiology of celiac disease
Image by Lecturio.Celiac disease may present in infancy or in the 3rd to 4th decades of life.
| Manifestations | Laboratory finding |
|---|---|
| Steatorrhea Steatorrhea A condition that is characterized by chronic fatty diarrhea, a result of abnormal digestion and/or intestinal absorption of fats. Diarrhea (bulky, foul-smelling, light-colored stool) | Increased fecal fat due to fat malabsorption Malabsorption General term for a group of malnutrition syndromes caused by failure of normal intestinal absorption of nutrients. Malabsorption and Maldigestion |
| Diarrhea Diarrhea Diarrhea is defined as ≥ 3 watery or loose stools in a 24-hour period. There are a multitude of etiologies, which can be classified based on the underlying mechanism of disease. The duration of symptoms (acute or chronic) and characteristics of the stools (e.g., watery, bloody, steatorrheic, mucoid) can help guide further diagnostic evaluation. Diarrhea (increased fecal content) | Increased stool osmolality Osmolality Plasma osmolality refers to the combined concentration of all solutes in the blood. Renal Sodium and Water Regulation gap due to unabsorbed fats Fats The glyceryl esters of a fatty acid, or of a mixture of fatty acids. They are generally odorless, colorless, and tasteless if pure, but they may be flavored according to origin. Fats are insoluble in water, soluble in most organic solvents. They occur in animal and vegetable tissue and are generally obtained by boiling or by extraction under pressure. They are important in the diet (dietary fats) as a source of energy. Energy Homeostasis and carbohydrates Carbohydrates A class of organic compounds composed of carbon, hydrogen, and oxygen in a ratio of cn(H2O)n. The largest class of organic compounds, including starch; glycogen; cellulose; polysaccharides; and simple monosaccharides. Basics of Carbohydrates |
| Weight loss Weight loss Decrease in existing body weight. Bariatric Surgery/ failure to thrive Failure to Thrive Failure to thrive (FTT), or faltering growth, describes suboptimal weight gain and growth in children. The majority of cases are due to inadequate caloric intake; however, genetic, infectious, and oncological etiologies are also common. Failure to Thrive/ muscle wasting Muscle Wasting Duchenne Muscular Dystrophy | Decreased D-xylose absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption due to inability to absorb any food content |
| Bleeding/repeated ecchymosis Ecchymosis Extravasation of blood into the skin, resulting in a nonelevated, rounded or irregular, blue or purplish patch, larger than a petechia. Orbital Fractures | Prolonged PT/INR ( prothrombin time Prothrombin time Clotting time of plasma recalcified in the presence of excess tissue thromboplastin. Factors measured are fibrinogen; prothrombin; factor V; factor VII; and factor X. Hemostasis/ international normalized ratio International normalized ratio System established by the world health organization and the international committee on thrombosis and hemostasis for monitoring and reporting blood coagulation tests. Under this system, results are standardized using the international sensitivity index for the particular test reagent/instrument combination used. Hemostasis) due to inability to absorb vitamin K Vitamin K A lipid cofactor that is required for normal blood clotting. Several forms of vitamin K have been identified: vitamin K 1 (phytomenadione) derived from plants, vitamin K 2 (menaquinone) from bacteria, and synthetic naphthoquinone provitamins, vitamin K 3 (menadione). Vitamin k 3 provitamins, after being alkylated in vivo, exhibit the antifibrinolytic activity of vitamin k. Green leafy vegetables, liver, cheese, butter, and egg yolk are good sources of vitamin k. Fat-soluble Vitamins and their Deficiencies |
| Microcytic anemia Microcytic anemia Conditions in which there is a generalized increase in the iron stores of body tissues, particularly of liver and the mononuclear phagocyte system, without demonstrable tissue damage. The name refers to the presence of stainable iron in the tissue in the form of hemosiderin. Anemia: Overview and Types | Low ferritin Ferritin Iron-containing proteins that are widely distributed in animals, plants, and microorganisms. Their major function is to store iron in a nontoxic bioavailable form. Each ferritin molecule consists of ferric iron in a hollow protein shell (apoferritins) made of 24 subunits of various sequences depending on the species and tissue types. Hereditary Hemochromatosis due to inability to absorb iron Iron A metallic element with atomic symbol fe, atomic number 26, and atomic weight 55. 85. It is an essential constituent of hemoglobins; cytochromes; and iron-binding proteins. It plays a role in cellular redox reactions and in the transport of oxygen. Trace Elements |
| Macrocytic anemia Macrocytic anemia Anemia characterized by larger than normal erythrocytes, increased mean corpuscular volume (MCV) and increased mean corpuscular hemoglobin (mMCH). Anemia: Overview and Types | Low serum B12 or folic acid due to inability to absorb vitamin B12 and B9 |
| Bone Bone Bone is a compact type of hardened connective tissue composed of bone cells, membranes, an extracellular mineralized matrix, and central bone marrow. The 2 primary types of bone are compact and spongy. Bones: Structure and Types pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways/fractures on minimal trauma | Osteopenia Osteopenia Osteoporosis on plain film and osteoporosis Osteoporosis Osteoporosis refers to a decrease in bone mass and density leading to an increased number of fractures. There are 2 forms of osteoporosis: primary, which is commonly postmenopausal or senile; and secondary, which is a manifestation of immobilization, underlying medical disorders, or long-term use of certain medications. Osteoporosis on DEXA DEXA Osteoporosis ( dual-energy X-ray absorptiometry Dual-Energy X-Ray Absorptiometry Osteoporosis) due to inability to absorb calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes and vitamin D Vitamin D A vitamin that includes both cholecalciferols and ergocalciferols, which have the common effect of preventing or curing rickets in animals. It can also be viewed as a hormone since it can be formed in skin by action of ultraviolet rays upon the precursors, 7-dehydrocholesterol and ergosterol, and acts on vitamin D receptors to regulate calcium in opposition to parathyroid hormone. Fat-soluble Vitamins and their Deficiencies |
| Milk intolerance | Abnormal lactose tolerance Tolerance Pharmacokinetics and Pharmacodynamics test due to inability to absorb lactose |
| Edema Edema Edema is a condition in which excess serous fluid accumulates in the body cavity or interstitial space of connective tissues. Edema is a symptom observed in several medical conditions. It can be categorized into 2 types, namely, peripheral (in the extremities) and internal (in an organ or body cavity). Edema | Decreased serum protein and albumin Albumin Serum albumin from humans. It is an essential carrier of both endogenous substances, such as fatty acids and bilirubin, and of xenobiotics in the blood. Liver Function Tests due to inability to absorb amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids from the diet |
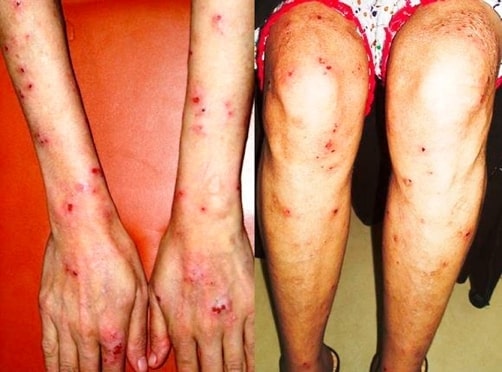
Dermatitis herpetiformis rash involving the extensor surface of the forearms, hands, and lower limbs in a patient with celiac disease
Image: “Skin lesions on dorsum of hand and legs” by Department of Surgery, The Aga Khan University Hospital (Stadium Road), Karachi (74800), Pakistan. License: CC BY 3.0Celiac disease is also associated with:
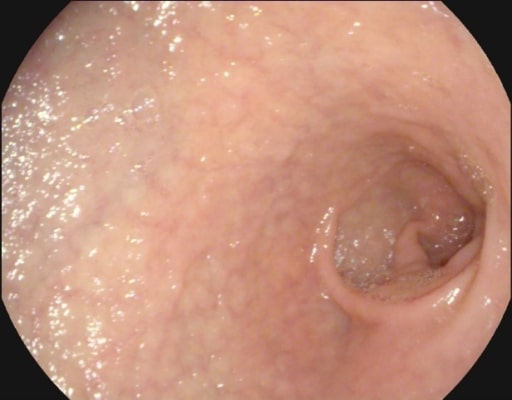
Mucosal atrophy and submucosal vascularity seen on endoscopy in a patient with celiac disease
Image: “Atrophy with visible vessel pattern in the duodenal bulb” by “Dr. Carol Davila” Central Military University Emergency Hospital, Bucharest, Romania ; “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania. License: CC BY 2.0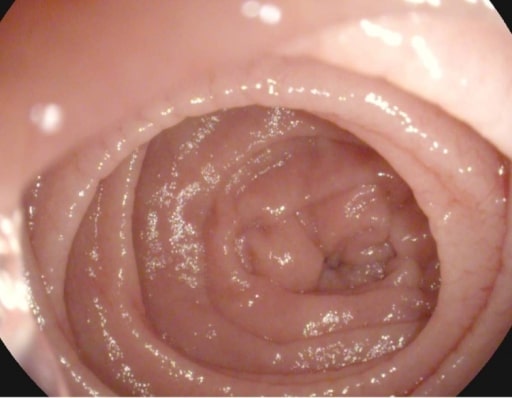
Scalloping of the Kerckring folds in a patient with celiac disease
Image: “Atrophy with visible vessel pattern in the duodenal bulb” by “Dr. Carol Davila” Central Military University Emergency Hospital, Bucharest, Romania; “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania. License: CC BY 2.0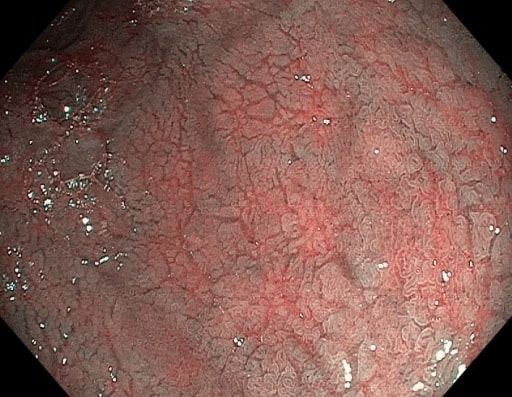
Mucosal fissures and prominent submucosal vessels seen on endoscopy in a patient with celiac disease
Image: “NBI” by “Dr. Carol Davila” Central Military University Emergency Hospital, Bucharest, Romania. License: CC BY 2.0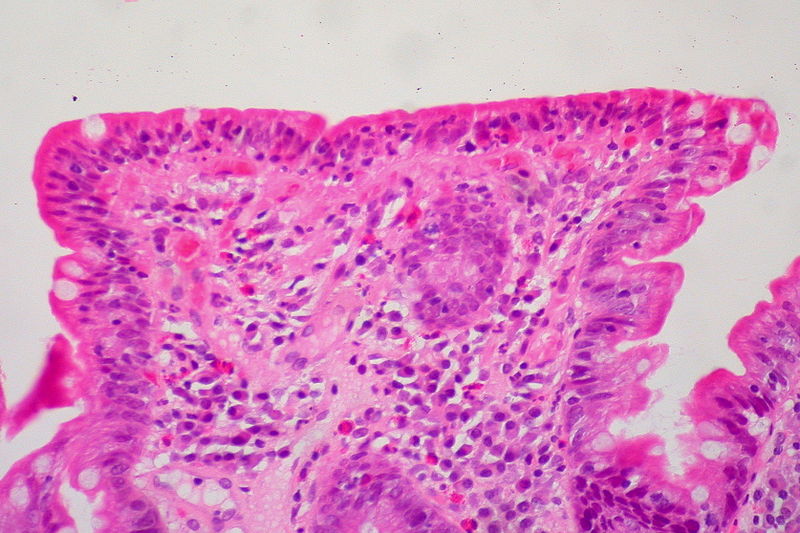
Small intestine biopsy showing crowding of lymphocytes (blue cells), loss of villi, and deepening (hyperplasia) of crypts
Image: “Celiac Sprue, Small Bowel Biopsy” by Ed Uthman from Houston, TX, USA. License: CC BY 2.0