Antiplatelet agents are medications that inhibit platelet aggregation Platelet aggregation The attachment of platelets to one another. This clumping together can be induced by a number of agents (e.g., thrombin; collagen) and is part of the mechanism leading to the formation of a thrombus. Hemostasis, a critical step in the formation of the initial platelet plug Platelet plug Hemostasis. Abnormal, or inappropriate, platelet aggregation Platelet aggregation The attachment of platelets to one another. This clumping together can be induced by a number of agents (e.g., thrombin; collagen) and is part of the mechanism leading to the formation of a thrombus. Hemostasis is a key step in the pathophysiology of arterial ischemic events. The primary categories of antiplatelet agents include aspirin Aspirin The prototypical analgesic used in the treatment of mild to moderate pain. It has anti-inflammatory and antipyretic properties and acts as an inhibitor of cyclooxygenase which results in the inhibition of the biosynthesis of prostaglandins. Aspirin also inhibits platelet aggregation and is used in the prevention of arterial and venous thrombosis. Nonsteroidal Antiinflammatory Drugs (NSAIDs), ADP inhibitors, phosphodiesterase/ adenosine Adenosine A nucleoside that is composed of adenine and d-ribose. Adenosine or adenosine derivatives play many important biological roles in addition to being components of DNA and RNA. Adenosine itself is a neurotransmitter. Class 5 Antiarrhythmic Drugs uptake inhibitors, and glycoprotein IIb/IIIa inhibitors. Common indications for antiplatelet agents include the treatment and prevention of ischemic heart disease Ischemic heart disease Coronary heart disease (CHD), or ischemic heart disease, describes a situation in which an inadequate supply of blood to the myocardium exists due to a stenosis of the coronary arteries, typically from atherosclerosis. Coronary Heart Disease and stroke, peripheral artery disease Peripheral artery disease Peripheral artery disease (PAD) is obstruction of the arterial lumen resulting in decreased blood flow to the distal limbs. The disease can be a result of atherosclerosis or thrombosis. Patients may be asymptomatic or have progressive claudication, skin discoloration, ischemic ulcers, or gangrene. Peripheral Artery Disease, and other conditions associated with a high risk for arterial thrombosis Arterial Thrombosis Hypercoagulable States.
Last updated: Jun 27, 2025
Antiplatelet agents are medications that inhibit platelet aggregation Platelet aggregation The attachment of platelets to one another. This clumping together can be induced by a number of agents (e.g., thrombin; collagen) and is part of the mechanism leading to the formation of a thrombus. Hemostasis, a critical step in the formation of the initial platelet plug Platelet plug Hemostasis.
Antiplatelets Antiplatelets Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. Heart Failure and Angina Medication are used in the treatment and/or prevention of arterial thrombosis Arterial Thrombosis Hypercoagulable States. Common indications include:
There are several primary classes of antiplatelet agents:
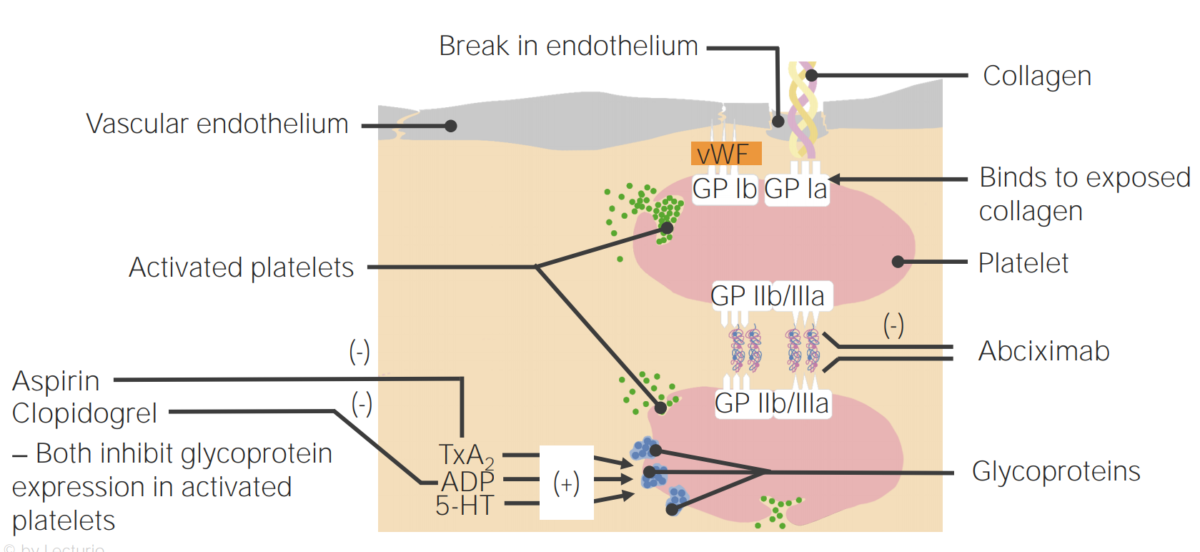
Mechanisms of action of antiplatelet agents
5-HT: 5-hydroxytryptamine
TxA2: thromboxane A2
VWF: von Willebrand factor
After endothelial injury occurs, exposure of the blood to the subendothelial Subendothelial Membranoproliferative Glomerulonephritis components triggers formation of the platelet plug Platelet plug Hemostasis. This process is known as primary hemostasis Primary hemostasis Hemostasis. (Formation of the fibrin Fibrin A protein derived from fibrinogen in the presence of thrombin, which forms part of the blood clot. Rapidly Progressive Glomerulonephritis clot via the coagulation cascade Coagulation cascade The coagulation cascade is a series of reactions that ultimately generates a strong, cross-linked fibrin clot. Hemostasis is secondary hemostasis Secondary hemostasis The coagulation cascade is a series of reactions that ultimately generates a strong, cross-linked fibrin clot. Hemostasis.)
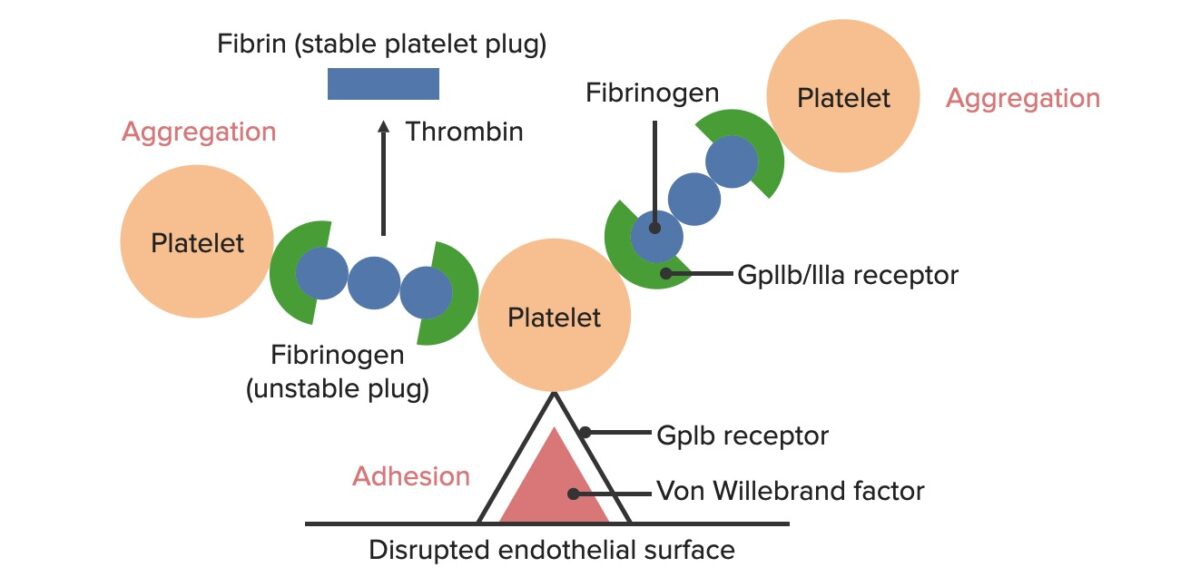
Formation of the temporary hemostatic plug:
The disrupted endothelial surface exposes VWF to the passing blood. Platelets bind to the VWF via their GpIb receptors and are activated. Platelet activation triggers them to secrete ADP, which stimulates the expression of the GpIIb/IIIa receptors on the platelets. The GpIIb/IIIa receptors bind to fibrinogen, which is able to bind a platelet on each end, causing platelets to aggregate. As more platelets are bound to one another, the platelet plug is generated. As the coagulation cascade is activated, thrombin converts the weaker fibrinogen into the stronger fibrin, creating a much more stable clot.
Exposure of the blood to subendothelial Subendothelial Membranoproliferative Glomerulonephritis components at the site of injury causes platelets Platelets Platelets are small cell fragments involved in hemostasis. Thrombopoiesis takes place primarily in the bone marrow through a series of cell differentiation and is influenced by several cytokines. Platelets are formed after fragmentation of the megakaryocyte cytoplasm. Platelets: Histology to adhere to that site.
Activated platelets Platelets Platelets are small cell fragments involved in hemostasis. Thrombopoiesis takes place primarily in the bone marrow through a series of cell differentiation and is influenced by several cytokines. Platelets are formed after fragmentation of the megakaryocyte cytoplasm. Platelets: Histology enhance further platelet adhesion Adhesion The process whereby platelets adhere to something other than platelets, e.g., collagen; basement membrane; microfibrils; or other ‘foreign’ surfaces. Coagulation Studies and aggregation Aggregation The attachment of platelets to one another. This clumping together can be induced by a number of agents (e.g., thrombin; collagen) and is part of the mechanism leading to the formation of a thrombus. Coagulation Studies and stimulate secretion Secretion Coagulation Studies of the platelet granules.
Platelets Platelets Platelets are small cell fragments involved in hemostasis. Thrombopoiesis takes place primarily in the bone marrow through a series of cell differentiation and is influenced by several cytokines. Platelets are formed after fragmentation of the megakaryocyte cytoplasm. Platelets: Histology contain granules, each of which releases a number of different substances when platelets Platelets Platelets are small cell fragments involved in hemostasis. Thrombopoiesis takes place primarily in the bone marrow through a series of cell differentiation and is influenced by several cytokines. Platelets are formed after fragmentation of the megakaryocyte cytoplasm. Platelets: Histology are activated. Functions of secreted substances include:
| Mechanism of Action |
|
|---|---|
| Physiologic effects |
|
| Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption |
|
| Distribution |
|
| Metabolism |
|
| Elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy |
|
| Specific indications |
|
| Specific contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation | Children < 16 years of age (risk of Reye’s syndrome if child develops a viral infection) |
| Complications/adverse effects |
|
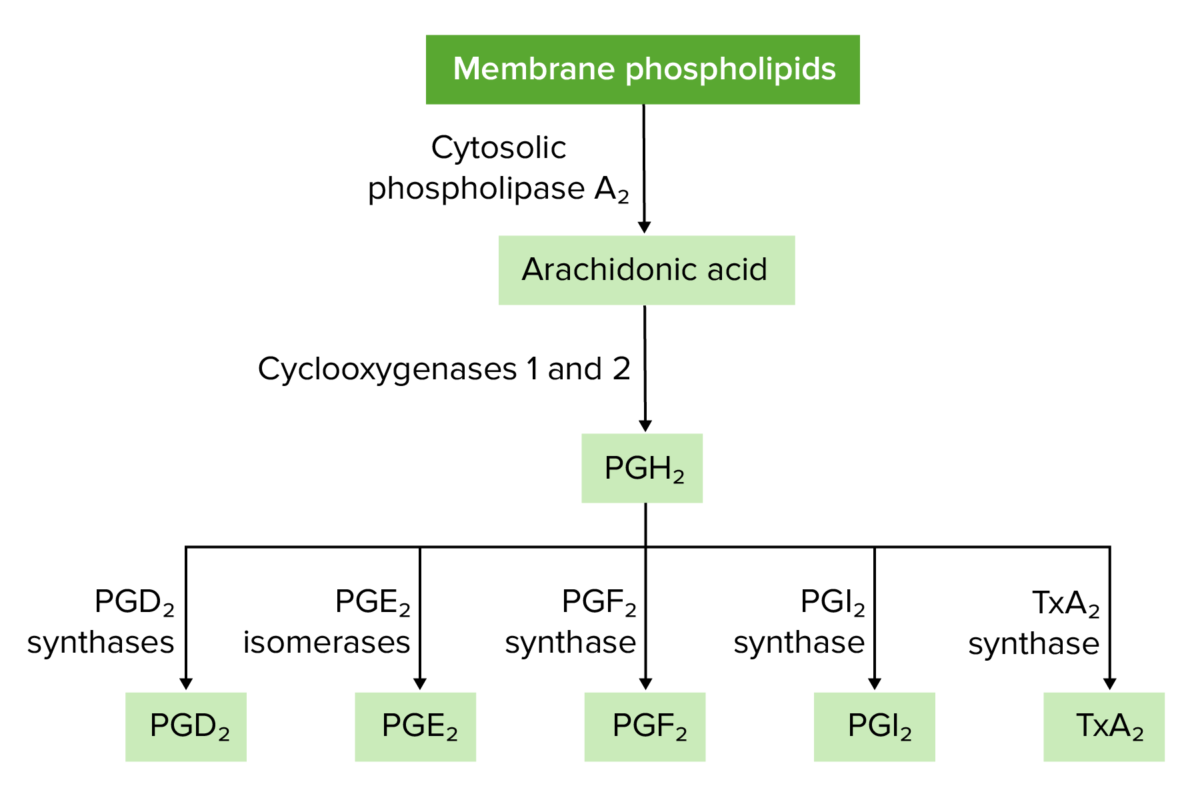
Overview of prostaglandin and thromboxane synthesis
PG: prostaglandin
| Agents |
|
|---|---|
| Mechanism of Action |
|
| Physiologic effects |
|
| Specific indications |
|
| Specific contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation | Prasugrel: history of an ischemic stroke Ischemic Stroke An ischemic stroke (also known as cerebrovascular accident) is an acute neurologic injury that occurs as a result of brain ischemia; this condition may be due to cerebral blood vessel occlusion by thrombosis or embolism, or rarely due to systemic hypoperfusion. Ischemic Stroke or TIA TIA Transient ischemic attack (TIA) is a temporary episode of neurologic dysfunction caused by ischemia without infarction that resolves completely when blood supply is restored. Transient ischemic attack is a neurologic emergency that warrants urgent medical attention. Transient Ischemic Attack (TIA) |
| Complications |
|
| Medication | Clopidogrel (Plavix®) | Prasugrel (Effient®) | Ticagrelor |
|---|---|---|---|
| Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption |
|
|
|
| Distribution | Highly protein-bound (98%) |
|
|
| Metabolism |
Metabolized in the
liver
Liver
The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood.
Liver: Anatomy via:
|
Hydrolyzed in the intestines and serum to an inactive thiolactone intermediate, which is then converted to an active metabolite via CYP3A4 CYP3A4 Class 3 Antiarrhythmic Drugs (Potassium Channel Blockers) and CYP2B6 oxidation | Metabolized by the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy to an active metabolite via CYP3A4 CYP3A4 Class 3 Antiarrhythmic Drugs (Potassium Channel Blockers) |
| Elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy |
|
|
|
| Agents | Dipyridamole Dipyridamole A phosphodiesterase inhibitor that blocks uptake and metabolism of adenosine by erythrocytes and vascular endothelial cells. Dipyridamole also potentiates the antiaggregating action of prostacyclin. Phosphodiesterase Inhibitors (Persantine®) | Cilostazol Cilostazol A quinoline and tetrazole derivative that acts as a phosphodiesterase type 3 inhibitor, with anti-platelet and vasodilating activity. It is used in the treatment of peripheral vascular diseases; ischemic heart disease; and in the prevention of stroke. Phosphodiesterase Inhibitors |
|---|---|---|
| Mechanism of Action |
Dual mechanisms of action:
|
|
| Physiologic effects |
|
|
| Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption |
|
|
| Distribution |
|
Protein binding: 95% |
| Metabolism |
|
|
| Elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy |
|
|
| Specific indications |
|
Intermittent claudication Intermittent claudication A symptom complex characterized by pain and weakness in skeletal muscle group associated with exercise, such as leg pain and weakness brought on by walking. Such muscle limpness disappears after a brief rest and is often relates to arterial stenosis; muscle ischemia; and accumulation of lactate. Thromboangiitis Obliterans (Buerger Disease) |
| Specific contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation |
|
Heart failure Heart Failure A heterogeneous condition in which the heart is unable to pump out sufficient blood to meet the metabolic need of the body. Heart failure can be caused by structural defects, functional abnormalities (ventricular dysfunction), or a sudden overload beyond its capacity. Chronic heart failure is more common than acute heart failure which results from sudden insult to cardiac function, such as myocardial infarction. Total Anomalous Pulmonary Venous Return (TAPVR) |
| Complications |
|
May induce tachycardia Tachycardia Abnormally rapid heartbeat, usually with a heart rate above 100 beats per minute for adults. Tachycardia accompanied by disturbance in the cardiac depolarization (cardiac arrhythmia) is called tachyarrhythmia. Sepsis in Children, tachyarrhythmias, and/or hypotension Hypotension Hypotension is defined as low blood pressure, specifically < 90/60 mm Hg, and is most commonly a physiologic response. Hypotension may be mild, serious, or life threatening, depending on the cause. Hypotension |
| Adverse effects |
|
|
The 2 primary GPIIb/IIIa inhibitors are eptifibatide (Integrilin®) and tirofiban (Aggrastat®). Abciximab is a monoclonal antibody that is also in this category; however, it is no longer available in the United States.
| Agents | Eptifibatide (Integrilin®) | Tirofiban (Aggrastat®) |
|---|---|---|
| Mechanism of Action | Binds to and reversibly inhibits the GPIIb/IIIa receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors | |
| Physiologic effects | Prevents GPIIb/IIIa receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors from binding fibrinogen Fibrinogen Plasma glycoprotein clotted by thrombin, composed of a dimer of three non-identical pairs of polypeptide chains (alpha, beta, gamma) held together by disulfide bonds. Fibrinogen clotting is a sol-gel change involving complex molecular arrangements: whereas fibrinogen is cleaved by thrombin to form polypeptides a and b, the proteolytic action of other enzymes yields different fibrinogen degradation products. Hemostasis and thus prevents platelet aggregation Platelet aggregation The attachment of platelets to one another. This clumping together can be induced by a number of agents (e.g., thrombin; collagen) and is part of the mechanism leading to the formation of a thrombus. Hemostasis | |
| Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption |
|
|
| Distribution |
|
|
| Metabolism | Negligible | |
| Elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy |
|
|
| Specific indications |
|
|
| Specific contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation | Severe thrombocytopenia Severe Thrombocytopenia Thrombocytopenia | |
| Complications |
|
|
Some of the most common therapeutic uses of antiplatelet agents include: