Paracoccidioidomycosis (PCM) is an endemic fungal infection caused by Paracoccidioides brasiliensis complex and P. lutzii. The fungus is geographically distributed across Mexico and South and Central America. Transmission is by inhalation, and most infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease are asymptomatic. The fungus infects the lungs Lungs Lungs are the main organs of the respiratory system. Lungs are paired viscera located in the thoracic cavity and are composed of spongy tissue. The primary function of the lungs is to oxygenate blood and eliminate CO2. Lungs: Anatomy and then spreads to the skin Skin The skin, also referred to as the integumentary system, is the largest organ of the body. The skin is primarily composed of the epidermis (outer layer) and dermis (deep layer). The epidermis is primarily composed of keratinocytes that undergo rapid turnover, while the dermis contains dense layers of connective tissue. Skin: Structure and Functions, mucous membranes, and other parts of the body. Primary infection Primary infection Herpes Simplex Virus 1 and 2 is typically self-limited. However, if infection is not contained by the host, and/or the patient is not treated, life-threatening complications can ensue. Acute infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease typically affect younger populations, with findings of lymphadenopathy Lymphadenopathy Lymphadenopathy is lymph node enlargement (> 1 cm) and is benign and self-limited in most patients. Etiologies include malignancy, infection, and autoimmune disorders, as well as iatrogenic causes such as the use of certain medications. Generalized lymphadenopathy often indicates underlying systemic disease. Lymphadenopathy, hepatosplenomegaly Hepatosplenomegaly Cytomegalovirus, skin Skin The skin, also referred to as the integumentary system, is the largest organ of the body. The skin is primarily composed of the epidermis (outer layer) and dermis (deep layer). The epidermis is primarily composed of keratinocytes that undergo rapid turnover, while the dermis contains dense layers of connective tissue. Skin: Structure and Functions lesions, and signs of bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis dysfunction. More commonly, chronic paracoccidioidomycosis is seen, especially in men. Chronic paracoccidioidomycosis represents reactivation Reactivation Herpes Simplex Virus 1 and 2 of the infection, presenting with lung disease or disseminated infection. Diagnosis is by microscopy, histopathology, culture, and serology Serology The study of serum, especially of antigen-antibody reactions in vitro. Yellow Fever Virus. Itraconazole Itraconazole A triazole antifungal agent that inhibits cytochrome p-450-dependent enzymes required for ergosterol synthesis. Azoles is typically used for mild to moderate disease and amphotericin B Amphotericin B Macrolide antifungal antibiotic produced by streptomyces nodosus obtained from soil of the orinoco river region of venezuela. Polyenes for severe and/or CNS disease.
Last updated: Mar 19, 2025
Morphology:
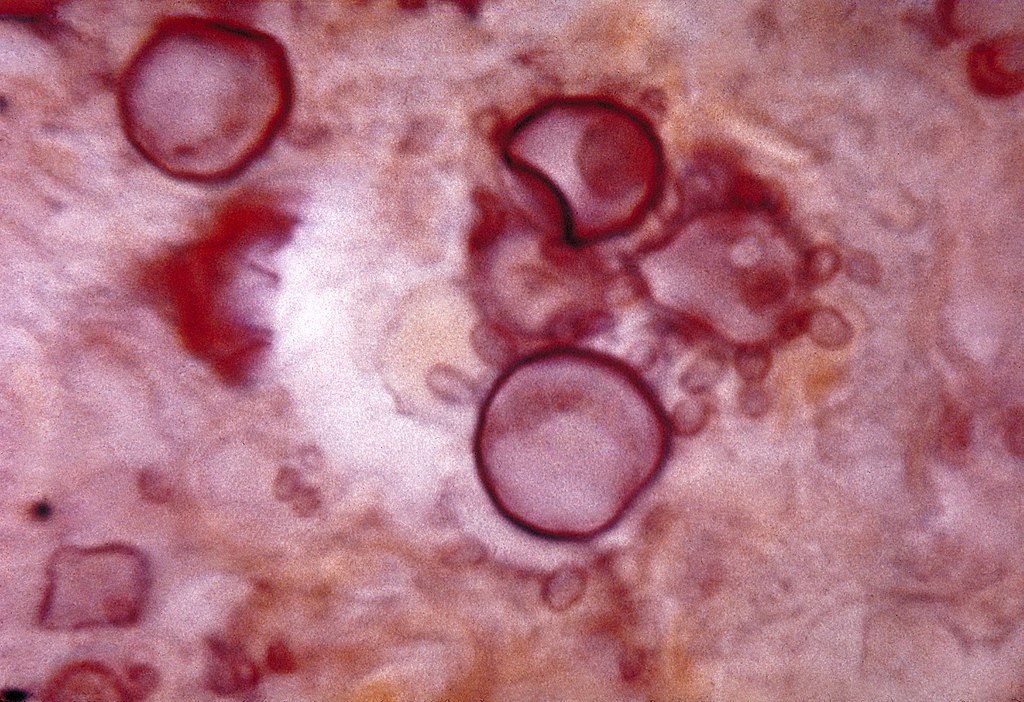
Histopathology of Paracoccidioides:
Cells with budding daughter cells (blastoconidia) seen in tissue, like a ship’s steering wheel (captain’s or pilot’s wheel)
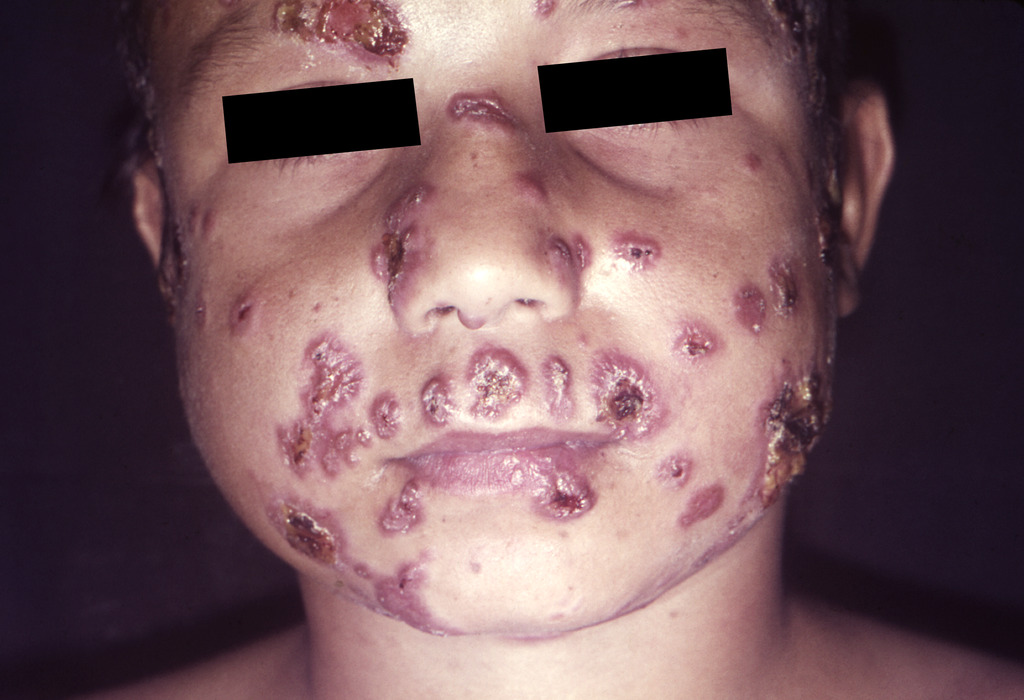
Paracoccidioidomycosis-caused facial lesions in a Brazilian child.
Image: “Paracoccidioidomycosis lesions” by CDC/Dr. Martins Castro. License: Public Domain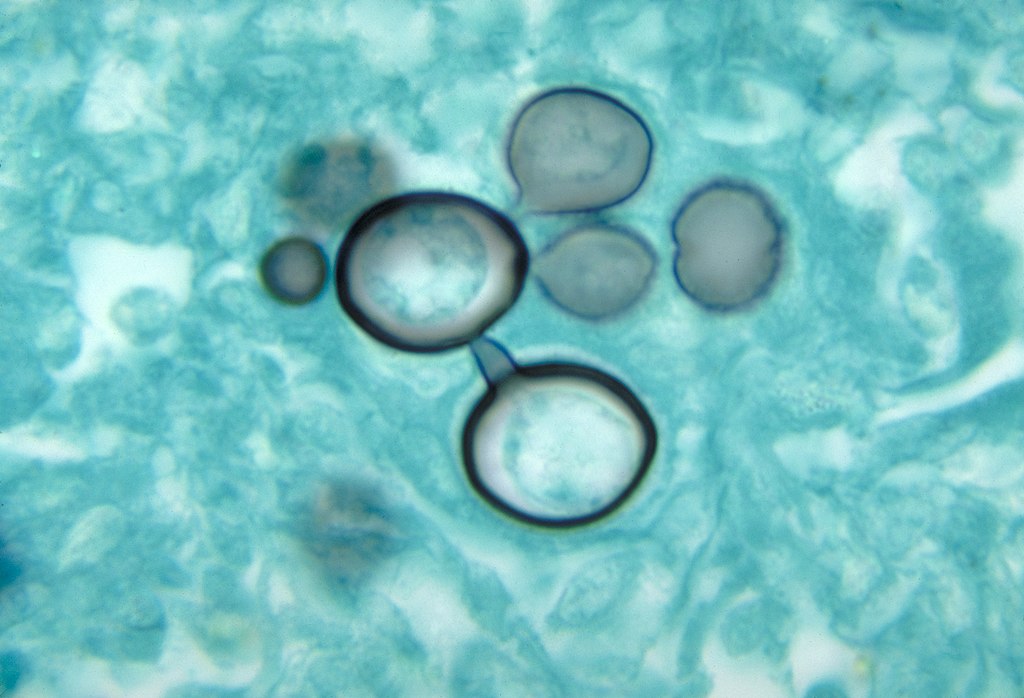
Histopathology of paracoccidioidomycosis
Image: “Histopathology of paracoccidiodomycoisis” by CDC. License: Public Domain
A culture of Paracoccidioides brasiliensis during its yeast phase
Image: “A culture of Paracoccidioides brasiliensis during its yeast phase” by CDC. License: Public Domain