Caustic agents are acidic or alkaline substances that damage tissues severely if ingested. Alkali ingestion typically damages the esophagus Esophagus The esophagus is a muscular tube-shaped organ of around 25 centimeters in length that connects the pharynx to the stomach. The organ extends from approximately the 6th cervical vertebra to the 11th thoracic vertebra and can be divided grossly into 3 parts: the cervical part, the thoracic part, and the abdominal part. Esophagus: Anatomy via liquefactive necrosis Liquefactive Necrosis Cell Injury and Death, whereas acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance cause more severe gastric injury leading to coagulative necrosis Coagulative Necrosis Cell Injury and Death. Ingestion of large volumes and high concentrations of caustic agents can lead to severe and extensive injuries. Additionally, aspiration affects the laryngeal and tracheobronchial structures. Signs and symptoms include oral pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways, burns Burns A burn is a type of injury to the skin and deeper tissues caused by exposure to heat, electricity, chemicals, friction, or radiation. Burns are classified according to their depth as superficial (1st-degree), partial-thickness (2nd-degree), full-thickness (3rd-degree), and 4th-degree burns. Burns, dysphagia Dysphagia Dysphagia is the subjective sensation of difficulty swallowing. Symptoms can range from a complete inability to swallow, to the sensation of solids or liquids becoming "stuck." Dysphagia is classified as either oropharyngeal or esophageal, with esophageal dysphagia having 2 sub-types: functional and mechanical. Dysphagia, vomiting Vomiting The forcible expulsion of the contents of the stomach through the mouth. Hypokalemia, and abdominal pain Abdominal Pain Acute Abdomen. Severe injuries can present with shock Shock Shock is a life-threatening condition associated with impaired circulation that results in tissue hypoxia. The different types of shock are based on the underlying cause: distributive (↑ cardiac output (CO), ↓ systemic vascular resistance (SVR)), cardiogenic (↓ CO, ↑ SVR), hypovolemic (↓ CO, ↑ SVR), obstructive (↓ CO), and mixed. Types of Shock, abdominal rigidity Abdominal Rigidity Acute Abdomen, respiratory distress, and/or altered mental status Altered Mental Status Sepsis in Children. Diagnosis is based on laboratory tests, abdominal and chest imaging, and endoscopy Endoscopy Procedures of applying endoscopes for disease diagnosis and treatment. Endoscopy involves passing an optical instrument through a small incision in the skin i.e., percutaneous; or through a natural orifice and along natural body pathways such as the digestive tract; and/or through an incision in the wall of a tubular structure or organ, i.e. Transluminal, to examine or perform surgery on the interior parts of the body. Gastroesophageal Reflux Disease (GERD) within 24 hours (if without contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation) to determine the extent of damage. Management involves stabilizing the cardiorespiratory status, decontamination, and supportive therapy. Severe injury may require surgery.
Last updated: Feb 19, 2025
Caustics and corrosives cause tissue injury by a chemical reaction.
Injury by alkaline chemicals is overall more toxic than that caused by acidic substances:
Caustic ingestion:
Common acid-containing products:
Common alkali-containing products:
Tissue injury occurs by changing the ionized state and structure of molecules, thereby disrupting the covalent bonds.
To help recall the damage caused by alkalis and acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance, the following mnemonic may be used:
Alkaline = liquefactive necrosis Necrosis The death of cells in an organ or tissue due to disease, injury or failure of the blood supply. Ischemic Cell Damage
Acidic = coagulation necrosis Necrosis The death of cells in an organ or tissue due to disease, injury or failure of the blood supply. Ischemic Cell Damage
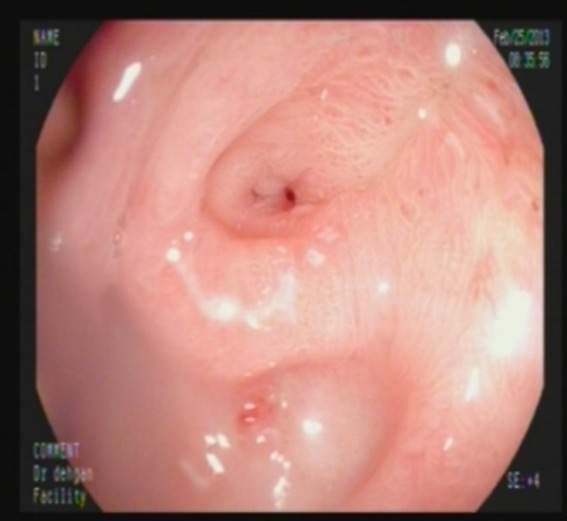
Endoscopic image of pyloric stenosis: 1 month after alkali ingestion
Image: “Pyloric stenosis after 1 month” by Shiraz Transplant Research Center, Gastroenterohepatology Research Center, Nemazee Teaching Hospital, Shiraz University of Medical Sciences, School of Medicine, Shiraz, Iran. License: CC BY 3.0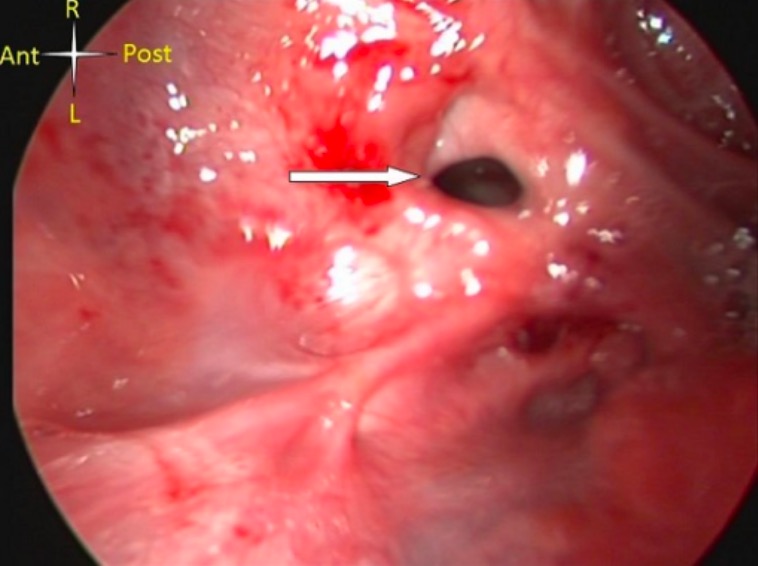
Severe caustic injury of the hypopharynx (arrow points to laryngeal opening)
Image: “Severe caustic injury of the pharynx” by National Cardiothoracic Centre, Korle Bu Teaching Hospital, Accra, Ghana. License: CC BY 2.0Asymptomatic patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship without significant ingestion or oral burns Burns A burn is a type of injury to the skin and deeper tissues caused by exposure to heat, electricity, chemicals, friction, or radiation. Burns are classified according to their depth as superficial (1st-degree), partial-thickness (2nd-degree), full-thickness (3rd-degree), and 4th-degree burns. Burns can be safely discharged.
Management of symptomatic patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship and individuals with significant ingestion includes the following: