Restrictive cardiomyopathy Cardiomyopathy Cardiomyopathy refers to a group of myocardial diseases associated with structural changes of the heart muscles (myocardium) and impaired systolic and/or diastolic function in the absence of other heart disorders (coronary artery disease, hypertension, valvular disease, and congenital heart disease). Cardiomyopathy: Overview and Types (RCM) is a fairly uncommon condition characterized by progressive stiffening of the cardiac muscle Cardiac muscle The muscle tissue of the heart. It is composed of striated, involuntary muscle cells connected to form the contractile pump to generate blood flow. Muscle Tissue: Histology, which causes impaired relaxation and refilling of the heart during diastole Diastole Post-systolic relaxation of the heart, especially the heart ventricles. Cardiac Cycle, resulting in diastolic dysfunction and eventual heart failure Heart Failure A heterogeneous condition in which the heart is unable to pump out sufficient blood to meet the metabolic need of the body. Heart failure can be caused by structural defects, functional abnormalities (ventricular dysfunction), or a sudden overload beyond its capacity. Chronic heart failure is more common than acute heart failure which results from sudden insult to cardiac function, such as myocardial infarction. Total Anomalous Pulmonary Venous Return (TAPVR). It most often occurs secondary to scarring Scarring Inflammation, damage, and/or infiltration of the heart muscle, with amyloidosis Amyloidosis Amyloidosis is a disease caused by abnormal extracellular tissue deposition of fibrils composed of various misfolded low-molecular-weight protein subunits. These proteins are frequently byproducts of other pathological processes (e.g., multiple myeloma). Amyloidosis being the most common cause. Infrequently, it may be idiopathic Idiopathic Dermatomyositis or inherited. Signs and symptoms include shortness of breath Shortness of breath Dyspnea is the subjective sensation of breathing discomfort. Dyspnea is a normal manifestation of heavy physical or psychological exertion, but also may be caused by underlying conditions (both pulmonary and extrapulmonary). Dyspnea, low exercise tolerance Tolerance Pharmacokinetics and Pharmacodynamics, fatigue Fatigue The state of weariness following a period of exertion, mental or physical, characterized by a decreased capacity for work and reduced efficiency to respond to stimuli. Fibromyalgia, and peripheral edema Peripheral edema Peripheral edema is the swelling of the lower extremities, namely, legs, feet, and ankles. Edema. Diagnosis is made through clinical suspicion and confirmed through ECG ECG An electrocardiogram (ECG) is a graphic representation of the electrical activity of the heart plotted against time. Adhesive electrodes are affixed to the skin surface allowing measurement of cardiac impulses from many angles. The ECG provides 3-dimensional information about the conduction system of the heart, the myocardium, and other cardiac structures. Electrocardiogram (ECG), X-ray X-ray Penetrating electromagnetic radiation emitted when the inner orbital electrons of an atom are excited and release radiant energy. X-ray wavelengths range from 1 pm to 10 nm. Hard x-rays are the higher energy, shorter wavelength x-rays. Soft x-rays or grenz rays are less energetic and longer in wavelength. The short wavelength end of the x-ray spectrum overlaps the gamma rays wavelength range. The distinction between gamma rays and x-rays is based on their radiation source. Pulmonary Function Tests, echocardiography Echocardiography Ultrasonic recording of the size, motion, and composition of the heart and surrounding tissues. The standard approach is transthoracic. Tricuspid Valve Atresia (TVA), and cardiac MRI Cardiac MRI Imaging of the Heart and Great Vessels. Treatment includes medications for heart failure Heart Failure A heterogeneous condition in which the heart is unable to pump out sufficient blood to meet the metabolic need of the body. Heart failure can be caused by structural defects, functional abnormalities (ventricular dysfunction), or a sudden overload beyond its capacity. Chronic heart failure is more common than acute heart failure which results from sudden insult to cardiac function, such as myocardial infarction. Total Anomalous Pulmonary Venous Return (TAPVR), implantable devices such as pacemakers and cardioverter–defibrillators, and heart transplantation Heart Transplantation The transference of a heart from one human or animal to another. Organ Transplantation in refractory cases.
Last updated: May 16, 2024
Restrictive cardiomyopathy Cardiomyopathy Cardiomyopathy refers to a group of myocardial diseases associated with structural changes of the heart muscles (myocardium) and impaired systolic and/or diastolic function in the absence of other heart disorders (coronary artery disease, hypertension, valvular disease, and congenital heart disease). Cardiomyopathy: Overview and Types (RCM) is a disease of the heart muscle characterized by decreased compliance Compliance Distensibility measure of a chamber such as the lungs (lung compliance) or bladder. Compliance is expressed as a change in volume per unit change in pressure. Veins: Histology of the ventricles, nondilated heart muscle, and diastolic dysfunction (impaired filling of the ventricles).
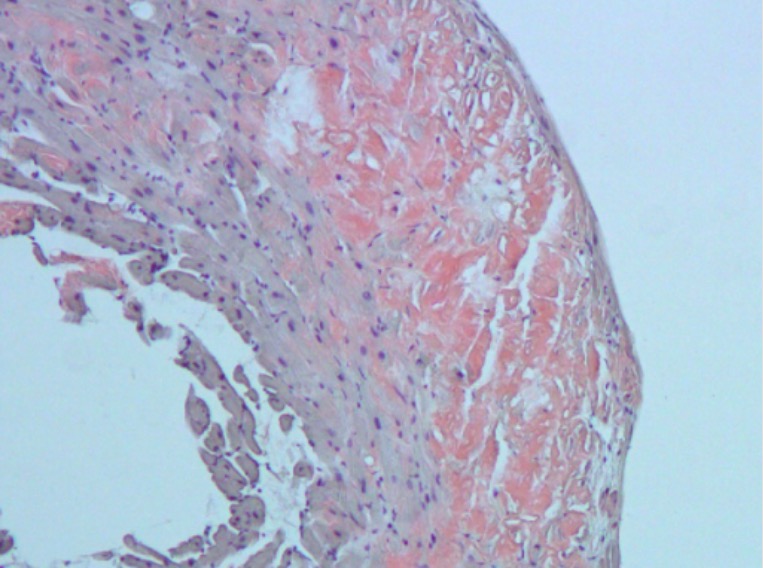
Congo red staining of myocardium affected by amyloidosis:
In cardiac amyloidosis, the myocardium contains large patches of hyaline material that stains with Congo red. The infiltration of the myocardium makes it stiff, causing restrictive cardiomyopathy.
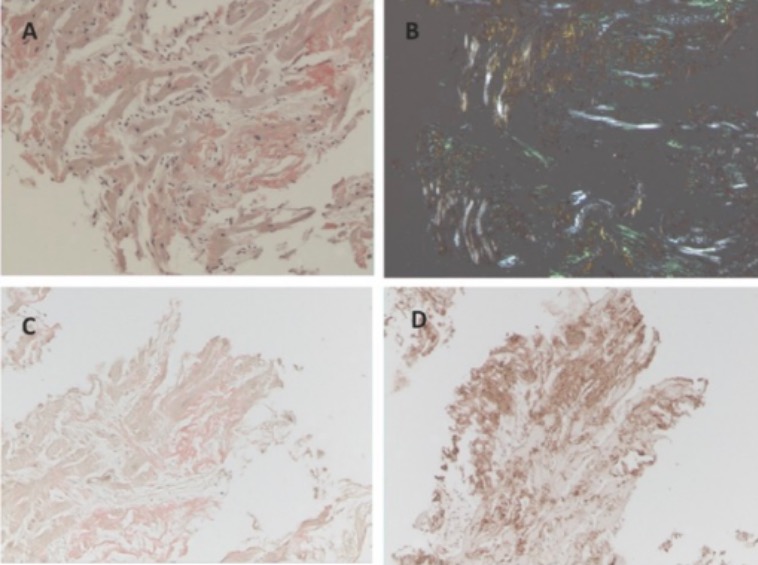
An endomyocardial biopsy of a patient with cardiac amyloidosis stained as follows:
A: Congo red only
B: Apple-green birefringence under polarized light
C: Congo red with lambda overlay (negative)
D: Congo red with kappa overlay (positive)
Presentation is similar to that seen with right heart failure Heart Failure A heterogeneous condition in which the heart is unable to pump out sufficient blood to meet the metabolic need of the body. Heart failure can be caused by structural defects, functional abnormalities (ventricular dysfunction), or a sudden overload beyond its capacity. Chronic heart failure is more common than acute heart failure which results from sudden insult to cardiac function, such as myocardial infarction. Total Anomalous Pulmonary Venous Return (TAPVR).
Jugular venous distension:
Elevated filling level of jugular vein can be a nonspecific finding in heart failure.
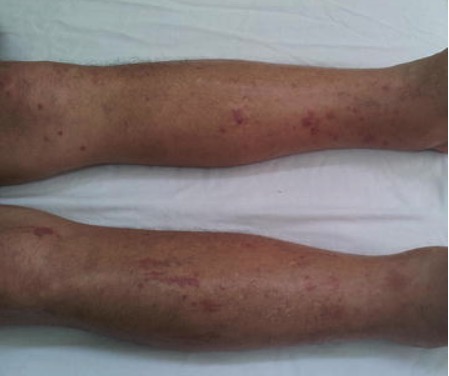
Patient presenting with pitting edema of the lower limbs, ecchymosis, and petechiae:
Pitting edema of the lower extremities is a nonspecific symptom of multiple etiologies and is due to the accumulation of fluid in the interstitial space of dependent areas, leading to swelling.
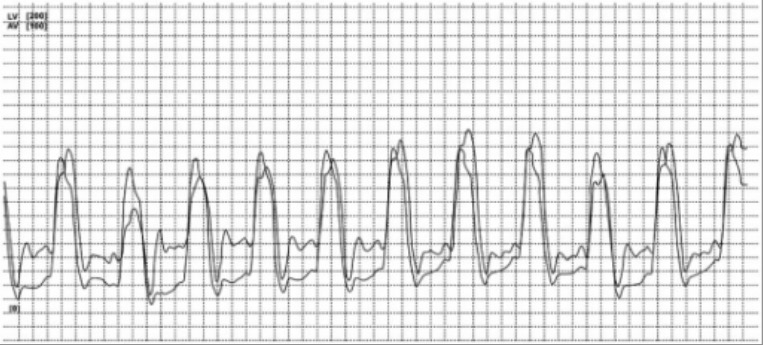
Patient with cardiac amyloidosis:
Right and left ventricular tracing with concordant change in pressures during expiration and inspiration confirming the diagnosis of restrictive cardiomyopathy
Cardiac MRI Cardiac MRI Imaging of the Heart and Great Vessels with gadolinium Gadolinium An element of the rare earth family of metals. It has the atomic symbol gd, atomic number 64, and atomic weight 157. 25. Its oxide is used in the control rods of some nuclear reactors. Magnetic Resonance Imaging (MRI) enhancement can identify and determine extent of:
Treatment is aimed at managing heart failure Heart Failure A heterogeneous condition in which the heart is unable to pump out sufficient blood to meet the metabolic need of the body. Heart failure can be caused by structural defects, functional abnormalities (ventricular dysfunction), or a sudden overload beyond its capacity. Chronic heart failure is more common than acute heart failure which results from sudden insult to cardiac function, such as myocardial infarction. Total Anomalous Pulmonary Venous Return (TAPVR) and underlying secondary causes.
Goals of treatment:
Medication:
Surgical intervention: