Severe congenital neutropenia Neutropenia Neutrophils are an important component of the immune system and play a significant role in the eradication of infections. Low numbers of circulating neutrophils, referred to as neutropenia, predispose the body to recurrent infections or sepsis, though patients can also be asymptomatic. Neutropenia (SCN) affects myelopoiesis Myelopoiesis Formation of myeloid cells from the pluripotent hematopoietic stem cells in the bone marrow via myeloid stem cells. Myelopoiesis generally refers to the production of leukocytes in blood, such as monocytes and granulocytes. This process also produces precursor cells for macrophage and dendritic cells found in the lymphoid tissue. White Myeloid Cells: Histology and has many different subtypes. SCN manifests in infancy with life-threatening bacterial infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease. The treatment proven to be effective is the administration of granulocyte colony-stimulating factor Granulocyte colony-stimulating factor A glycoprotein of mw 25 kda containing internal disulfide bonds. It induces the survival, proliferation, and differentiation of neutrophilic granulocyte precursor cells and functionally activates mature blood neutrophils. Among the family of colony-stimulating factors, G-CSF is the most potent inducer of terminal differentiation to granulocytes and macrophages of leukemic myeloid cell lines. White Myeloid Cells: Histology, which elevates the decreased neutrophil count. Kostmann disease (SCN3) has an autosomal recessive inheritance Autosomal recessive inheritance Autosomal Recessive and Autosomal Dominant Inheritance pattern, whereas the most common subtype, SCN1, has autosomal dominant inheritance Autosomal dominant inheritance Autosomal Recessive and Autosomal Dominant Inheritance.
Last updated: Feb 25, 2025
In 50%–60% of cases, severe congenital neutropenia Neutropenia Neutrophils are an important component of the immune system and play a significant role in the eradication of infections. Low numbers of circulating neutrophils, referred to as neutropenia, predispose the body to recurrent infections or sepsis, though patients can also be asymptomatic. Neutropenia (SCN) is due to an autosomal dominant Autosomal dominant Autosomal inheritance, both dominant and recessive, refers to the transmission of genes from the 22 autosomal chromosomes. Autosomal dominant diseases are expressed when only 1 copy of the dominant allele is inherited. Autosomal Recessive and Autosomal Dominant Inheritance ELANE gene mutation Gene Mutation Myotonic Dystrophies. However, the initial mutation Mutation Genetic mutations are errors in DNA that can cause protein misfolding and dysfunction. There are various types of mutations, including chromosomal, point, frameshift, and expansion mutations. Types of Mutations described by Kostmann was in HAX1, which is inherited in an autosomal recessive Autosomal recessive Autosomal inheritance, both dominant and recessive, refers to the transmission of genes from the 22 autosomal chromosomes. Autosomal recessive diseases are only expressed when 2 copies of the recessive allele are inherited. Autosomal Recessive and Autosomal Dominant Inheritance pattern. X-linked recessive X-Linked Recessive Duchenne Muscular Dystrophy inheritance is due to mutations in the Wiskott-Aldrich syndrome Wiskott-Aldrich syndrome Wiskott-Aldrich syndrome (WAS), also known as eczema-thrombocytopenia-immunodeficiency syndrome, IMD2, or immunodeficiency 2, is a rare genetic mixed disorder of B- and T-cell deficiency that follows an X-linked recessive inheritance pattern. It is caused by a WAS gene mutation that leads to impaired actin cytoskeleton, phagocytosis and chemotaxis, impaired platelet development, and, in general, a loss of humoral and cellular responses. Wiskott-Aldrich Syndrome (WAS) gene Gene A category of nucleic acid sequences that function as units of heredity and which code for the basic instructions for the development, reproduction, and maintenance of organisms. Basic Terms of Genetics protein (WASP).
The following table summarizes each subtype of SCN, the genes Genes A category of nucleic acid sequences that function as units of heredity and which code for the basic instructions for the development, reproduction, and maintenance of organisms. DNA Types and Structure involved and their function, as well as their mode of inheritance.
| Type | Gene Gene A category of nucleic acid sequences that function as units of heredity and which code for the basic instructions for the development, reproduction, and maintenance of organisms. Basic Terms of Genetics mutated | Protein affected | Mode of inheritance |
|---|---|---|---|
| SCN1 | ELANE (19p13.3) | Neutrophil elastase Elastase A protease of broad specificity, obtained from dried pancreas. Molecular weight is approximately 25, 000. The enzyme breaks down elastin, the specific protein of elastic fibers, and digests other proteins such as fibrin, hemoglobin, and albumin. Proteins and Peptides ( mutation Mutation Genetic mutations are errors in DNA that can cause protein misfolding and dysfunction. There are various types of mutations, including chromosomal, point, frameshift, and expansion mutations. Types of Mutations leads to misfolded protein, which leads to increased apoptosis Apoptosis A regulated cell death mechanism characterized by distinctive morphologic changes in the nucleus and cytoplasm, including the endonucleolytic cleavage of genomic DNA, at regularly spaced, internucleosomal sites, I.e., DNA fragmentation. It is genetically-programmed and serves as a balance to mitosis in regulating the size of animal tissues and in mediating pathologic processes associated with tumor growth. Ischemic Cell Damage) | AD AD The term advance directive (AD) refers to treatment preferences and/or the designation of a surrogate decision-maker in the event that a person becomes unable to make medical decisions on their own behalf. Advance directives represent the ethical principle of autonomy and may take the form of a living will, health care proxy, durable power of attorney for health care, and/or a physician’s order for life-sustaining treatment. Advance Directives |
| SCN2 | GFI1 (1p22.1) | Repressor of transcriptional processes ( mutation Mutation Genetic mutations are errors in DNA that can cause protein misfolding and dysfunction. There are various types of mutations, including chromosomal, point, frameshift, and expansion mutations. Types of Mutations leads to loss of repression Repression Defense mechanisms involving approach and avoidance responses to threatening stimuli. The sensitizing process involves intellectualization in approaching or controlling the stimulus whereas repression involves unconscious denial in avoiding the stimulus. Defense Mechanisms) | AD AD The term advance directive (AD) refers to treatment preferences and/or the designation of a surrogate decision-maker in the event that a person becomes unable to make medical decisions on their own behalf. Advance directives represent the ethical principle of autonomy and may take the form of a living will, health care proxy, durable power of attorney for health care, and/or a physician’s order for life-sustaining treatment. Advance Directives |
| SCN3 | HAX1 (1q21.3) | HCLS1-associated protein X-1 (functions in control of apoptosis Apoptosis A regulated cell death mechanism characterized by distinctive morphologic changes in the nucleus and cytoplasm, including the endonucleolytic cleavage of genomic DNA, at regularly spaced, internucleosomal sites, I.e., DNA fragmentation. It is genetically-programmed and serves as a balance to mitosis in regulating the size of animal tissues and in mediating pathologic processes associated with tumor growth. Ischemic Cell Damage) | AR AR Aortic regurgitation (AR) is a cardiac condition characterized by the backflow of blood from the aorta to the left ventricle during diastole. Aortic regurgitation is associated with an abnormal aortic valve and/or aortic root stemming from multiple causes, commonly rheumatic heart disease as well as congenital and degenerative valvular disorders. Aortic Regurgitation |
| SCN4 | G6PC3 (17q21.31) | G6Pase— mutation Mutation Genetic mutations are errors in DNA that can cause protein misfolding and dysfunction. There are various types of mutations, including chromosomal, point, frameshift, and expansion mutations. Types of Mutations leads to abolished enzyme activity, aberrant glycosylation Glycosylation The chemical or biochemical addition of carbohydrate or glycosyl groups to other chemicals, especially peptides or proteins. Glycosyl transferases are used in this biochemical reaction. Post-translational Protein Processing, and enhanced apoptosis Apoptosis A regulated cell death mechanism characterized by distinctive morphologic changes in the nucleus and cytoplasm, including the endonucleolytic cleavage of genomic DNA, at regularly spaced, internucleosomal sites, I.e., DNA fragmentation. It is genetically-programmed and serves as a balance to mitosis in regulating the size of animal tissues and in mediating pathologic processes associated with tumor growth. Ischemic Cell Damage of myeloid cells Myeloid Cells The classes of bone marrow-derived blood cells in the monocytic series (monocytes and their precursors) and granulocytic series (granulocytes and their precursors). White Myeloid Cells: Histology | AR AR Aortic regurgitation (AR) is a cardiac condition characterized by the backflow of blood from the aorta to the left ventricle during diastole. Aortic regurgitation is associated with an abnormal aortic valve and/or aortic root stemming from multiple causes, commonly rheumatic heart disease as well as congenital and degenerative valvular disorders. Aortic Regurgitation |
| SCN5 | VPS45 (1q21.2) | Vesicle Vesicle Primary Skin Lesions mediated protein (controls vesicular trafficking) | AR AR Aortic regurgitation (AR) is a cardiac condition characterized by the backflow of blood from the aorta to the left ventricle during diastole. Aortic regurgitation is associated with an abnormal aortic valve and/or aortic root stemming from multiple causes, commonly rheumatic heart disease as well as congenital and degenerative valvular disorders. Aortic Regurgitation |
| SCNX | WAS (Xp11.23)—implicated in Wiskott-Aldrich syndrome Wiskott-Aldrich syndrome Wiskott-Aldrich syndrome (WAS), also known as eczema-thrombocytopenia-immunodeficiency syndrome, IMD2, or immunodeficiency 2, is a rare genetic mixed disorder of B- and T-cell deficiency that follows an X-linked recessive inheritance pattern. It is caused by a WAS gene mutation that leads to impaired actin cytoskeleton, phagocytosis and chemotaxis, impaired platelet development, and, in general, a loss of humoral and cellular responses. Wiskott-Aldrich Syndrome | WASP—regulator of actin Actin Filamentous proteins that are the main constituent of the thin filaments of muscle fibers. The filaments (known also as filamentous or f-actin) can be dissociated into their globular subunits; each subunit is composed of a single polypeptide 375 amino acids long. This is known as globular or g-actin. In conjunction with myosins, actin is responsible for the contraction and relaxation of muscle. Skeletal Muscle Contraction cytoskeleton Cytoskeleton The network of filaments, tubules, and interconnecting filamentous bridges which give shape, structure, and organization to the cytoplasm. The Cell: Cytosol and Cytoskeleton ( mutation Mutation Genetic mutations are errors in DNA that can cause protein misfolding and dysfunction. There are various types of mutations, including chromosomal, point, frameshift, and expansion mutations. Types of Mutations is a gain-of-function mutation Mutation Genetic mutations are errors in DNA that can cause protein misfolding and dysfunction. There are various types of mutations, including chromosomal, point, frameshift, and expansion mutations. Types of Mutations leading to loss of autoinhibition) | X-linked recessive X-Linked Recessive Duchenne Muscular Dystrophy |
Neutropenia Neutropenia Neutrophils are an important component of the immune system and play a significant role in the eradication of infections. Low numbers of circulating neutrophils, referred to as neutropenia, predispose the body to recurrent infections or sepsis, though patients can also be asymptomatic. Neutropenia may arise from any of 3 pathogenic pathways:
Hereditary:
Acquired:
Autoimmune:
Infectious:
Mixed: chronic benign Benign Fibroadenoma neutropenia Neutropenia Neutrophils are an important component of the immune system and play a significant role in the eradication of infections. Low numbers of circulating neutrophils, referred to as neutropenia, predispose the body to recurrent infections or sepsis, though patients can also be asymptomatic. Neutropenia
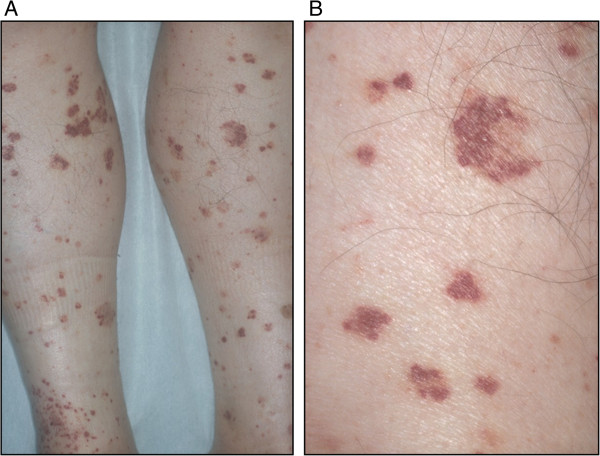
Petechial bleeds are 1 of the various clinical presentations in severe congenital neutropenia (SCN)
Image: “Purpura” by Oshikata C, Tsurikisawa N, Takigawa M, Omori T, Sugano S, Tsuburai T, Mitomi H, Takemura T, Akiyama K. License: CC BY 2.0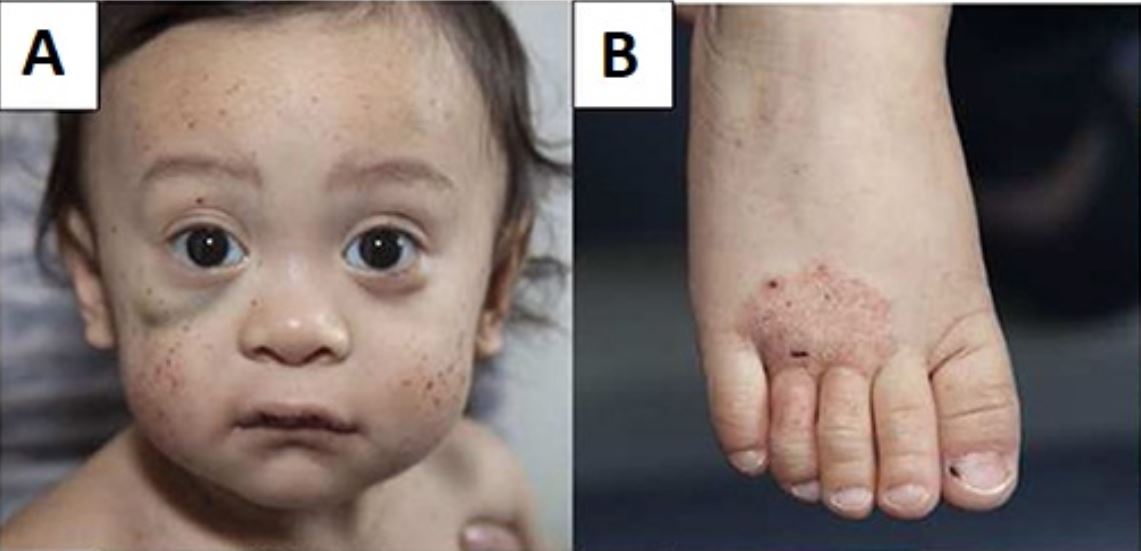
Young boy with Wiskott–Aldrich syndrome presenting with multiple facial petechiae and a hematoma under the right eye (A) and eczema of the foot (B)
Image: “Wiskott–Aldrich syndrome petechiae, hematoma and eczema” by Michael H. Albert and Alexandra F. Freeman. License: CC BY 4.0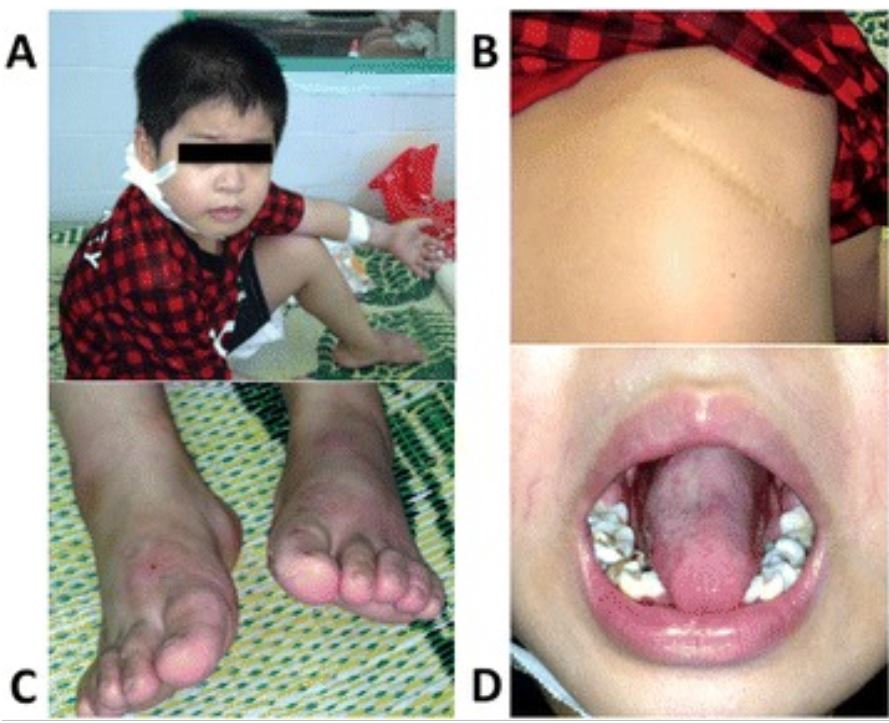
Severe congenital neutropenia caused by the ELANE gene mutation. Images show a number of lesions caused by infection.
A: Cutaneous abscess behind the right ear
B: Severe pneumonia requiring a pulmonary lobectomy
C: Fungal infection
D: Mouth ulcer
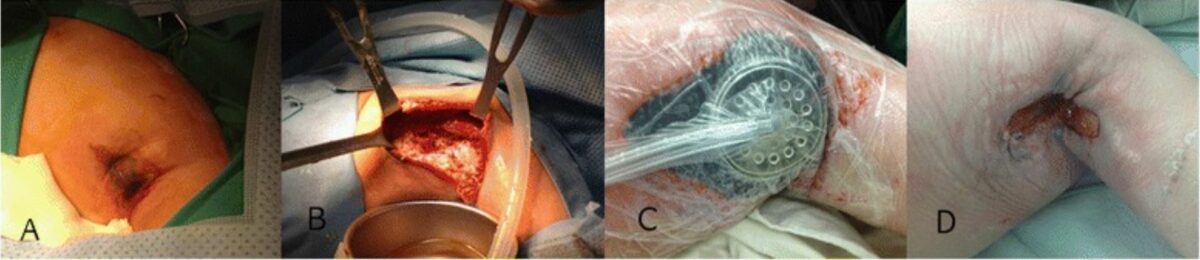
Cyclic neutropenia with a novel gene mutation presents with a necrotizing soft tissue infection and severe sepsis. Successful treatment of the axillary wound:
A: The initial lesion on the axilla comprised swollen edematous skin with a bluish change at the center.
B: Note the massive necrosis of the subcutaneous area, muscles, and fascia with contaminated discharge found at the initial surgery.
C: View after negative-pressure wound therapy
D: After 1 month, granulation tissue grew rapidly to enable primary repair.
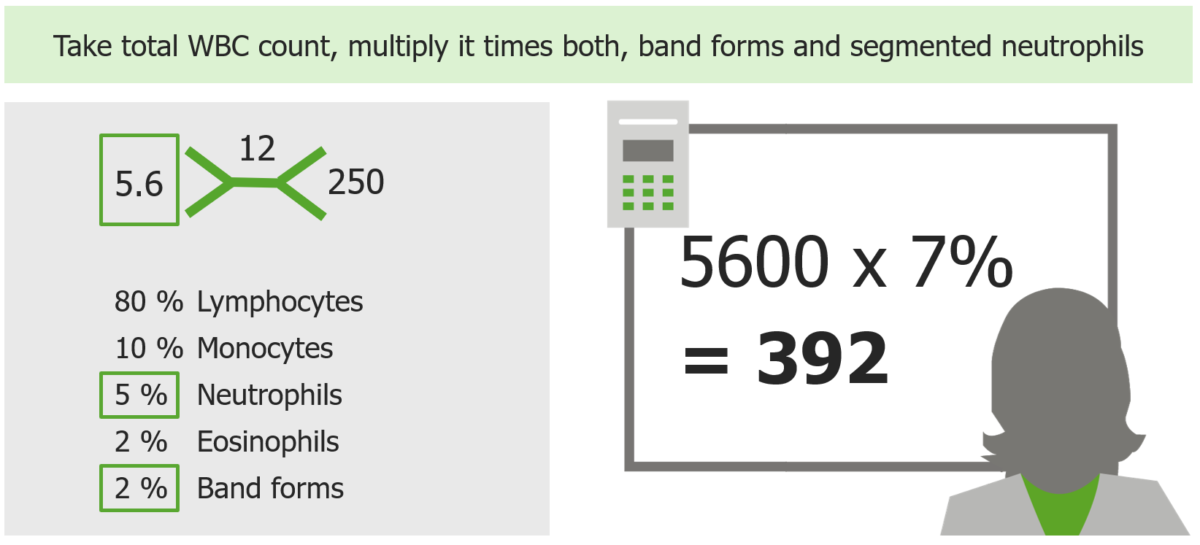
How to calculate ANC with example
Image by Lecturio. License: CC BY-NC-SA 4.0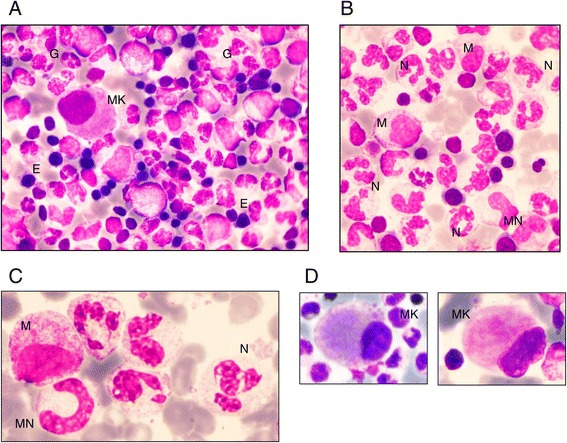
Bone marrow morphology in patients with G6PC3 congenital neutropenia:
A: Typical global bone marrow morphology: rich cellularity with predominant granulopoiesis (G), some erythroblasts (E), and 1 micromegakaryocyte (MK)
B: Predominant granulopoiesis with some myelocytes (M), very few metamyelocytes (MN), and a high number of mature neutrophils (N)
C: Details of neutrophils: hypersegmented appearance with thin opening between lobes and chromatin clumps
D: Examples of micromegakaryocytes
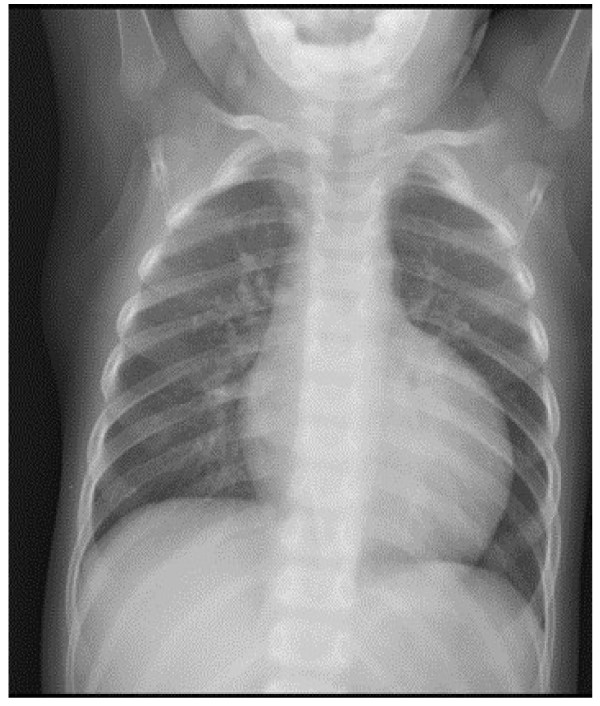
Chest X-ray showing severe cardiomegaly in a male patient with neutropenia and growth delay
Image: “Cardiomyopathy in a male patient with neutropenia and growth delay” by Folsi, V., et al. License: CC BY 4.0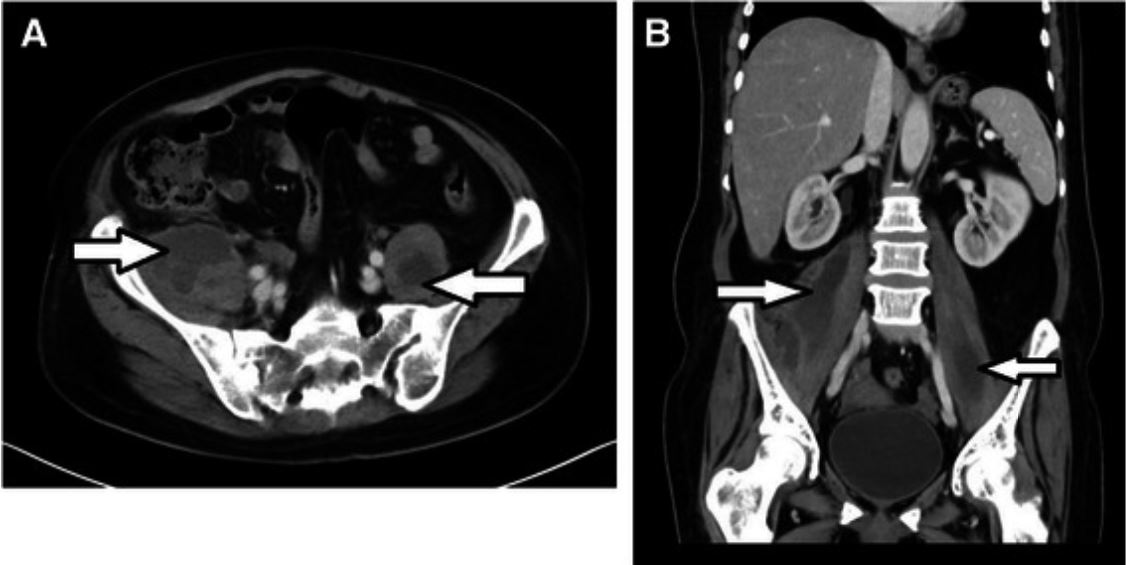
Bilateral primary psoas abscesses due to methicillin-resistant Staphylococcus aureus in a neutropenic patient.
Axial (A) and coronal (B) abdomen and pelvis CT with IV contrast showing bilateral psoas muscle abscesses (arrows)
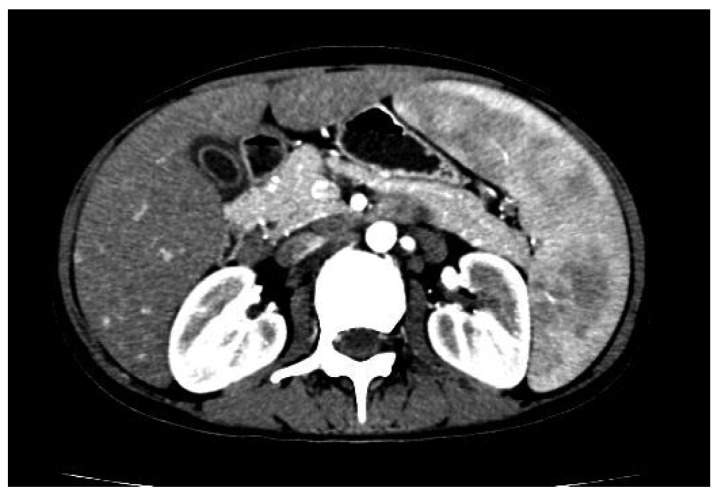
Felty syndrome (FS) is a specific subcategory of rheumatoid arthritis (RA) characterized by the triad of RA, severe extraarticular disease, and unexplained neutropenia.
The image shows an abdominal CT of a patient with FS demonstrating splenomegaly with heterogeneous attenuation due to angiographic contrast timing and multiple enlarged lymph nodes distributed along the retroperitoneal space, omental bursa, mesentery root, and surrounding hepatic hilum
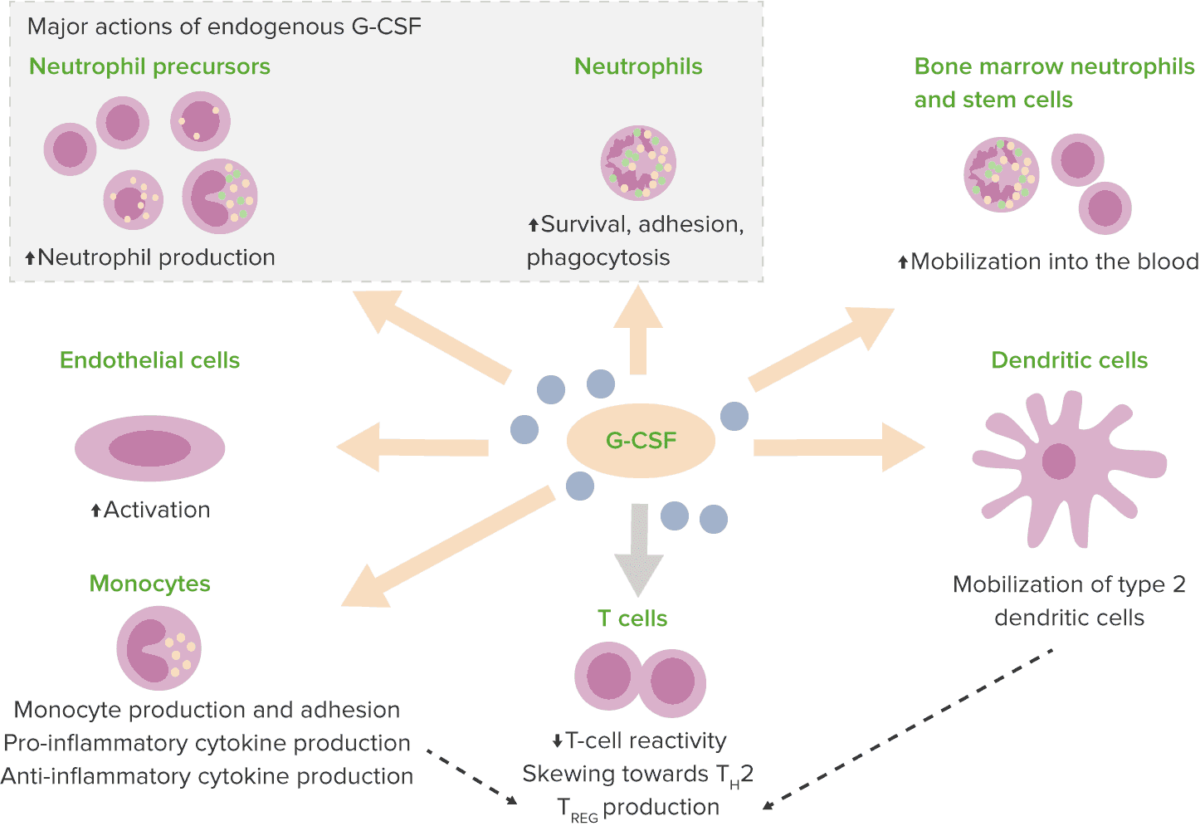
Effects of granulocyte-colony stimulating factor (G-CSF)
Th2: type 2 T helper cell
TREG: T regulatory cells