Local anesthetics Anesthetics Agents that are capable of inducing a total or partial loss of sensation, especially tactile sensation and pain. They may act to induce general anesthesia, in which an unconscious state is achieved, or may act locally to induce numbness or lack of sensation at a targeted site. Anesthesiology: History and Basic Concepts are a group of pharmacological agents that reversibly block the conduction of impulses in electrically excitable tissues. Local anesthetics Anesthetics Agents that are capable of inducing a total or partial loss of sensation, especially tactile sensation and pain. They may act to induce general anesthesia, in which an unconscious state is achieved, or may act locally to induce numbness or lack of sensation at a targeted site. Anesthesiology: History and Basic Concepts are used in clinical practice to induce a state of local or regional anesthesia Anesthesia A state characterized by loss of feeling or sensation. This depression of nerve function is usually the result of pharmacologic action and is induced to allow performance of surgery or other painful procedures. Anesthesiology: History and Basic Concepts by blocking sodium Sodium A member of the alkali group of metals. It has the atomic symbol na, atomic number 11, and atomic weight 23. Hyponatremia channels Channels The Cell: Cell Membrane and inhibiting the conduction of painful stimuli via afferent Afferent Neurons which conduct nerve impulses to the central nervous system. Nervous System: Histology nerves. The effects of local anesthetics Anesthetics Agents that are capable of inducing a total or partial loss of sensation, especially tactile sensation and pain. They may act to induce general anesthesia, in which an unconscious state is achieved, or may act locally to induce numbness or lack of sensation at a targeted site. Anesthesiology: History and Basic Concepts are influenced by their physicochemical properties such as lipid solubility and protein binding, as well as nerve anatomy and local tissue conditions. Adverse events, interactions, and contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation vary depending on the agent that is used.
Last updated: Nov 17, 2022
Chemical structure of cocaine
Image: “Cocaine” by Edgar181. License: Public Domain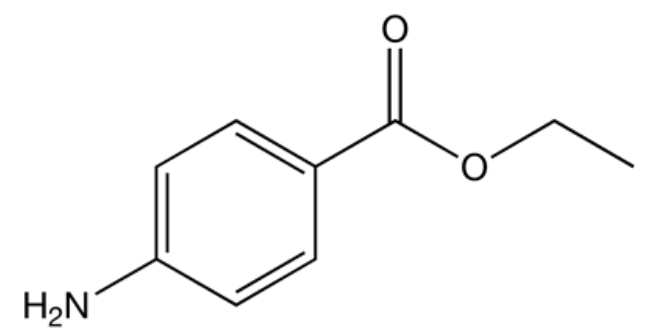
Chemical structure of benzocaine
Image: “Benzocaine” by Mykhal. License: Public Domain
Chemical structure of bupivacaine
Image: “Bupivacaine” by Mykhal. License: Public Domain| Agent | Half-life Half-Life The time it takes for a substance (drug, radioactive nuclide, or other) to lose half of its pharmacologic, physiologic, or radiologic activity. Pharmacokinetics and Pharmacodynamics (adults) | Onset of action | Metabolism | Excretion |
|---|---|---|---|---|
| Cocaine | 30 minutes to 1.5 hours | 15–30 seconds | Hydrolysis Hydrolysis The process of cleaving a chemical compound by the addition of a molecule of water. Proteins and Peptides: spontaneous and by plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products pseudocholinesterase; hepatic | Renal |
| Benzocaine | Short (exact numbers are unavailable) | 15–30 seconds | Hydrolysis Hydrolysis The process of cleaving a chemical compound by the addition of a molecule of water. Proteins and Peptides by plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products cholinesterase Cholinesterase Liver Function Tests; hepatic | Renal |
| Procaine | 7–8 minutes | 5–10 minutes | Hydrolysis Hydrolysis The process of cleaving a chemical compound by the addition of a molecule of water. Proteins and Peptides by plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products cholinesterase Cholinesterase Liver Function Tests | Renal |
| Lidocaine | 2 hours | 45–90 seconds | Hepatic (CYP1A2 and CYP3A4 CYP3A4 Class 3 Antiarrhythmic Drugs (Potassium Channel Blockers)) | Renal |
| Bupivacaine | 2–3 hours |
|
Hepatic | Renal |
Local anesthetics Anesthetics Agents that are capable of inducing a total or partial loss of sensation, especially tactile sensation and pain. They may act to induce general anesthesia, in which an unconscious state is achieved, or may act locally to induce numbness or lack of sensation at a targeted site. Anesthesiology: History and Basic Concepts can be classified into 2 structural groups:
Local anesthetics Anesthetics Agents that are capable of inducing a total or partial loss of sensation, especially tactile sensation and pain. They may act to induce general anesthesia, in which an unconscious state is achieved, or may act locally to induce numbness or lack of sensation at a targeted site. Anesthesiology: History and Basic Concepts can be classified on the basis of how they are administered.
| Esters | Amides | ||
|---|---|---|---|
| Topical agents | Parenteral agents | Intermediate acting | Long acting |
|
|
Lidocaine |
|
Local anesthetics Anesthetics Agents that are capable of inducing a total or partial loss of sensation, especially tactile sensation and pain. They may act to induce general anesthesia, in which an unconscious state is achieved, or may act locally to induce numbness or lack of sensation at a targeted site. Anesthesiology: History and Basic Concepts are chosen because of their onset of action, potency, and duration of action. The choice of a local anesthetic depends on the clinical needs, tolerance Tolerance Pharmacokinetics and Pharmacodynamics of the individual, and physician preference. Local anesthetics Anesthetics Agents that are capable of inducing a total or partial loss of sensation, especially tactile sensation and pain. They may act to induce general anesthesia, in which an unconscious state is achieved, or may act locally to induce numbness or lack of sensation at a targeted site. Anesthesiology: History and Basic Concepts are often used in combination with nonsteroidal anti-inflammatory drugs, opioids Opioids Opiates are drugs that are derived from the sap of the opium poppy. Opiates have been used since antiquity for the relief of acute severe pain. Opioids are synthetic opiates with properties that are substantially similar to those of opiates. Opioid Analgesics, and anticonvulsants.
| Agent | Effects |
|---|---|
| Cocaine |
|
| Benzocaine |
|
| Procaine | Hypersensitivity |
| Tetracaine |
|
| Lidocaine |
|
| Bupivacaine |
|
| Ropivacaine |
|