The carbapenems and aztreonam are both members of the bactericidal Bactericidal Penicillins beta-lactam Beta-Lactam Penicillins family of antibiotics (similar to penicillins Penicillins Beta-lactam antibiotics contain a beta-lactam ring as a part of their chemical structure. Drugs in this class include penicillin G and V, penicillinase-sensitive and penicillinase-resistant penicillins, cephalosporins, carbapenems, and aztreonam. Penicillins). They work by preventing bacteria Bacteria Bacteria are prokaryotic single-celled microorganisms that are metabolically active and divide by binary fission. Some of these organisms play a significant role in the pathogenesis of diseases. Bacteriology from producing their cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic, ultimately leading to bacterial cell death Cell death Injurious stimuli trigger the process of cellular adaptation, whereby cells respond to withstand the harmful changes in their environment. Overwhelmed adaptive mechanisms lead to cell injury. Mild stimuli produce reversible injury. If the stimulus is severe or persistent, injury becomes irreversible. Apoptosis is programmed cell death, a mechanism with both physiologic and pathologic effects. Cell Injury and Death. There are 4 available carbapenems, all ending in “-penem,” and 1 available monobactam, which is aztreonam. The carbapenems are all broad-spectrum Broad-Spectrum Fluoroquinolones antibiotics used for a variety of serious, often MDR and/or hospital-acquired infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease (HAIs) that can occur throughout the body. Aztreonam has a narrower spectrum and is typically used for aerobic gram-negative bacilli Bacilli Shigella infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease in patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship who have a serious beta-lactam Beta-Lactam Penicillins allergy Allergy An abnormal adaptive immune response that may or may not involve antigen-specific IgE Type I Hypersensitivity Reaction but require beta-lactam Beta-Lactam Penicillins therapy, as there is no significant cross-allergenicity between aztreonam and the other beta-lactams.
Last updated: Oct 6, 2022
Carbapenems and monobactams are both members of the beta-lactam Beta-Lactam Penicillins family of antibiotics. They are all cell-wall synthesis Synthesis Polymerase Chain Reaction (PCR) inhibitors. Members of the beta-lactam Beta-Lactam Penicillins family include:
Carbapenems consist of:
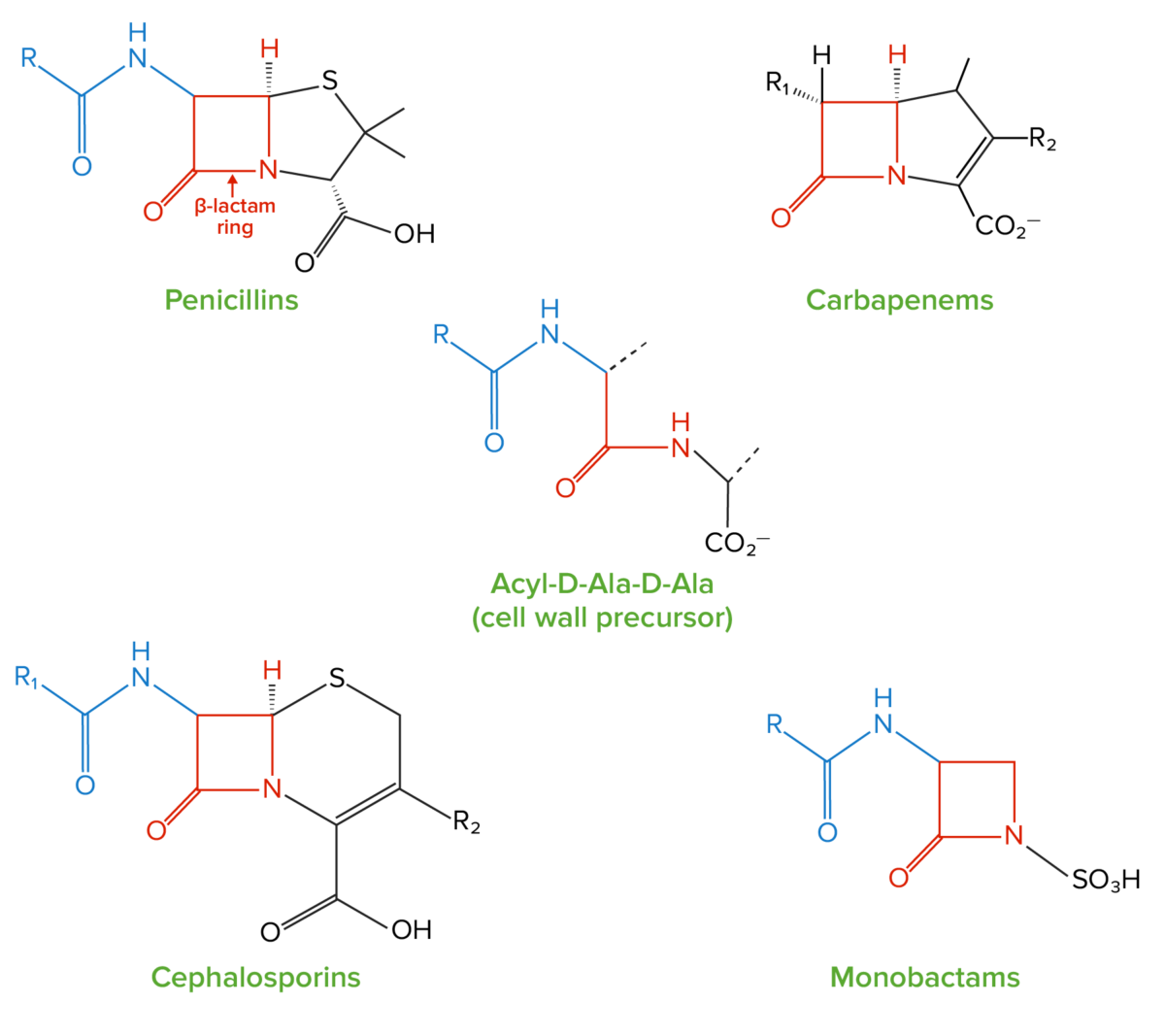
Structure of beta-lactams:
All beta-lactam antibiotics contain the same core 4-member “beta-lactam” ring (highlighted in red). This ring is responsible for the antibacterial properties of these medications because it is the region that binds to and inhibits the penicillin-binding proteins (PBPs). The PBPs catalyze formation of the cell wall by forming cross-links between peptide chains in peptidoglycan molecules; the PBPs form these cross-links between acyl-D-Ala-D-Ala peptides, which have a similar structure to the beta-lactam ring.
Monobactams are also beta-lactam Beta-Lactam Penicillins antibiotics. Their structure is different enough from penicillin Penicillin Rheumatic Fever that there is no cross-allergy with penicillins Penicillins Beta-lactam antibiotics contain a beta-lactam ring as a part of their chemical structure. Drugs in this class include penicillin G and V, penicillinase-sensitive and penicillinase-resistant penicillins, cephalosporins, carbapenems, and aztreonam. Penicillins. Azetreonam is the only marketed monobactam. Monobactam structures include:
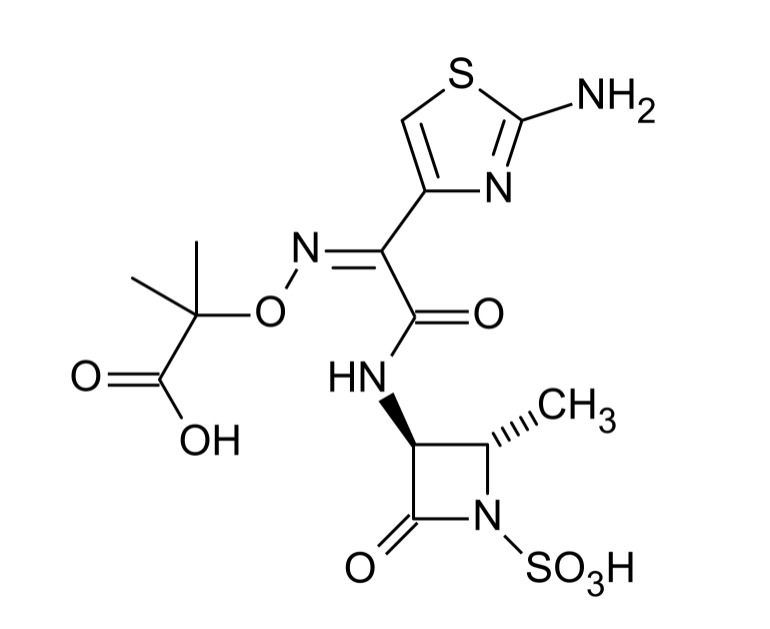
Structure of aztreonam, the only marketed monobactam in the United States
Image: “Chemical structure of aztreonam” by Mysid. License: Public DomainAll beta-lactams, including carbapenems and monobactams, work by inhibiting bacterial cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic synthesis Synthesis Polymerase Chain Reaction (PCR).
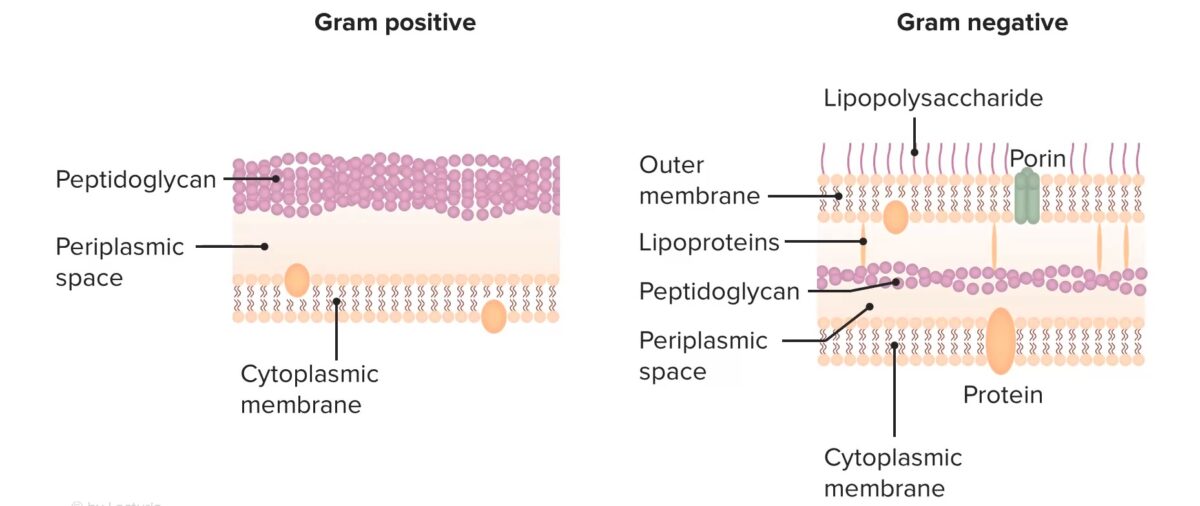
Structure of bacterial cell walls
Image by Lecturio. License: CC BY-NC-SA 4.0All beta-lactams work by irreversibly binding to and inhibiting the PBPs PBPs Bacterial proteins that share the property of binding irreversibly to penicillins and other antibacterial agents derived from lactams. The penicillin-binding proteins are primarily enzymes involved in cell wall biosynthesis including muramoylpentapeptide carboxypeptidase; peptide synthases; transpeptidases; and hexosyltransferases. Penicillins → beta-lactam Beta-Lactam Penicillins antibiotics inhibit cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic synthesis Synthesis Polymerase Chain Reaction (PCR)
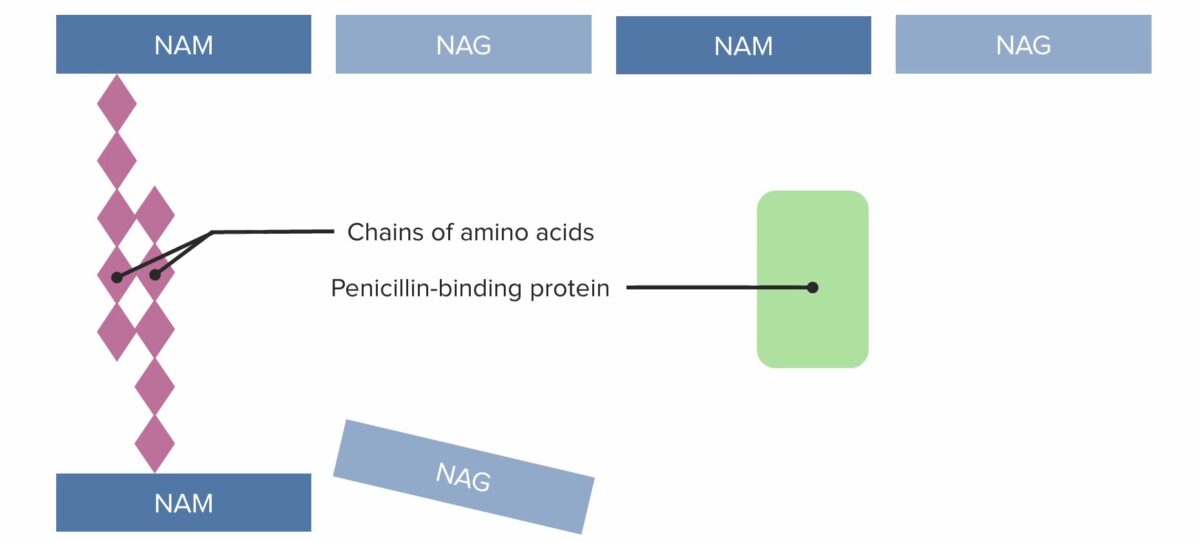
(1) Penicillin-binding protein (PBP) forming cross-linked bridges between adjacent peptidoglycan chains
Image by Lecturio. License: CC BY-NC-SA 4.0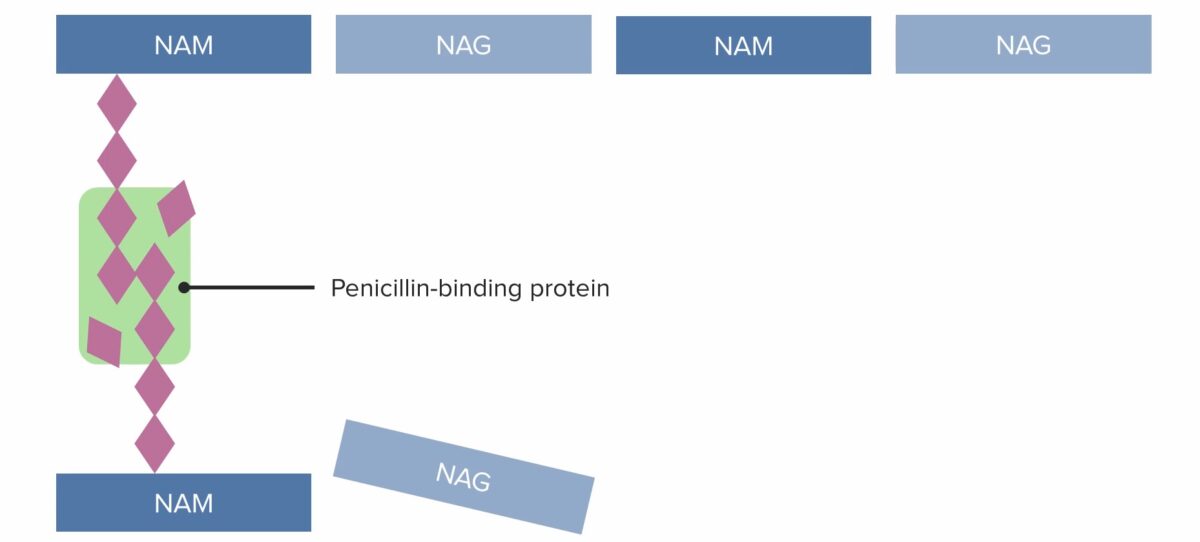
(2) Penicillin-binding protein (PBP) forming cross-linked bridges between adjacent peptidoglycan chains
Image by Lecturio. License: CC BY-NC-SA 4.0
(3) Penicillin-binding protein (PBP) forming cross-linked bridges between adjacent peptidoglycan chains
Image by Lecturio. License: CC BY-NC-SA 4.0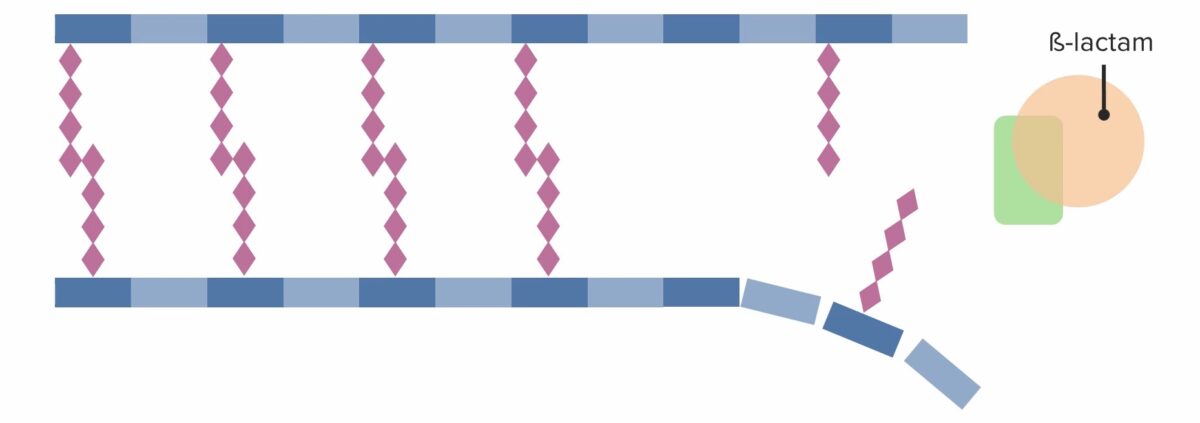
Presence of a beta-lactam antibiotic, which irreversibly binds to and inhibits the PBP, preventing it from forming new cross-links:
This effectively inhibits further cell wall synthesis, which ultimately leads to cell death.
Beta-lactams, including the carbapenems and monobactams, are bactericidal Bactericidal Penicillins (rather than bacteriostatic Bacteriostatic Sulfonamides and Trimethoprim).
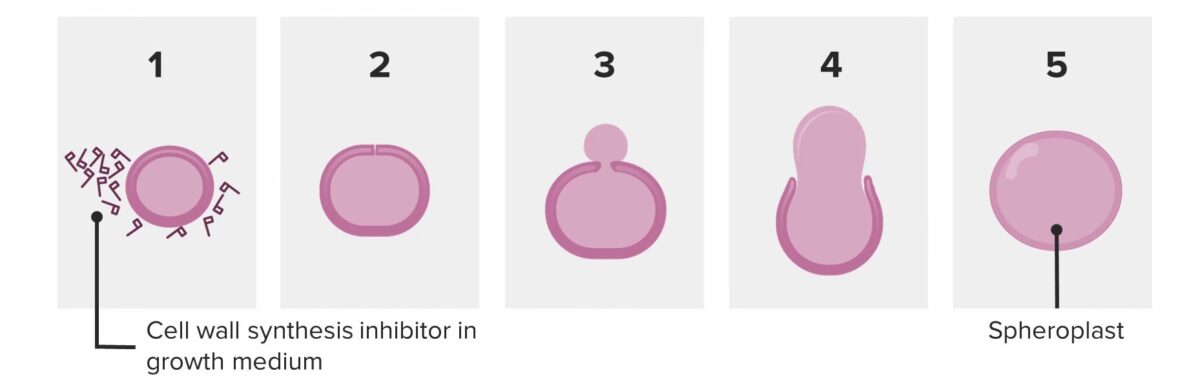
Bacterium attempting to divide in the presence of penicillin:
The bacterium ends up shedding its wall and becoming a spheroplast. The spheroplast is unable to survive and autocatalyzes (dies).
Bacteria Bacteria Bacteria are prokaryotic single-celled microorganisms that are metabolically active and divide by binary fission. Some of these organisms play a significant role in the pathogenesis of diseases. Bacteriology employ 3 primary mechanisms to resist beta-lactams:
Due to the increasing problem of resistance Resistance Physiologically, the opposition to flow of air caused by the forces of friction. As a part of pulmonary function testing, it is the ratio of driving pressure to the rate of air flow. Ventilation: Mechanics of Breathing from beta-lactamases, beta-lactamase Beta-Lactamase Penicillins inhibitors have been developed and are often combined with different beta-lactam Beta-Lactam Penicillins antibiotics. Available carbapenem and monobactam/ beta-lactamase Beta-Lactamase Penicillins inhibitor combinations include:
Antibiotics can be classified in several ways. One way is to classify them by mechanism of action:
| Mechanism | Classes of antibiotics |
|---|---|
| Bacterial cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic synthesis Synthesis Polymerase Chain Reaction (PCR) inhibitors |
|
| Bacterial protein synthesis Synthesis Polymerase Chain Reaction (PCR) inhibitors |
|
| Agents acting against DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure and/or folate Folate Folate and vitamin B12 are 2 of the most clinically important water-soluble vitamins. Deficiencies can present with megaloblastic anemia, GI symptoms, neuropsychiatric symptoms, and adverse pregnancy complications, including neural tube defects. Folate and Vitamin B12 |
|
| Antimycobacterial agents Antimycobacterial Agents Antimycobacterial agents represent a diverse group of compounds that have activity against mycobacterial infections, including tuberculosis, leprosy and Mycobacterium avium complex (MAC) disease. The 1st-line agents for tuberculosis are rifampin, isoniazid, pyrazinamide, and ethambutol. Antimycobacterial Drugs |
|
Different antibiotics have varying degrees of activity against different bacteria Bacteria Bacteria are prokaryotic single-celled microorganisms that are metabolically active and divide by binary fission. Some of these organisms play a significant role in the pathogenesis of diseases. Bacteriology. The table below outlines which antibiotics have activity against 3 important classes of bacteria Bacteria Bacteria are prokaryotic single-celled microorganisms that are metabolically active and divide by binary fission. Some of these organisms play a significant role in the pathogenesis of diseases. Bacteriology, including gram-positive Gram-Positive Penicillins cocci Cocci Bacteriology, gram-negative bacilli Bacilli Shigella, and anaerobes Anaerobes Lincosamides.
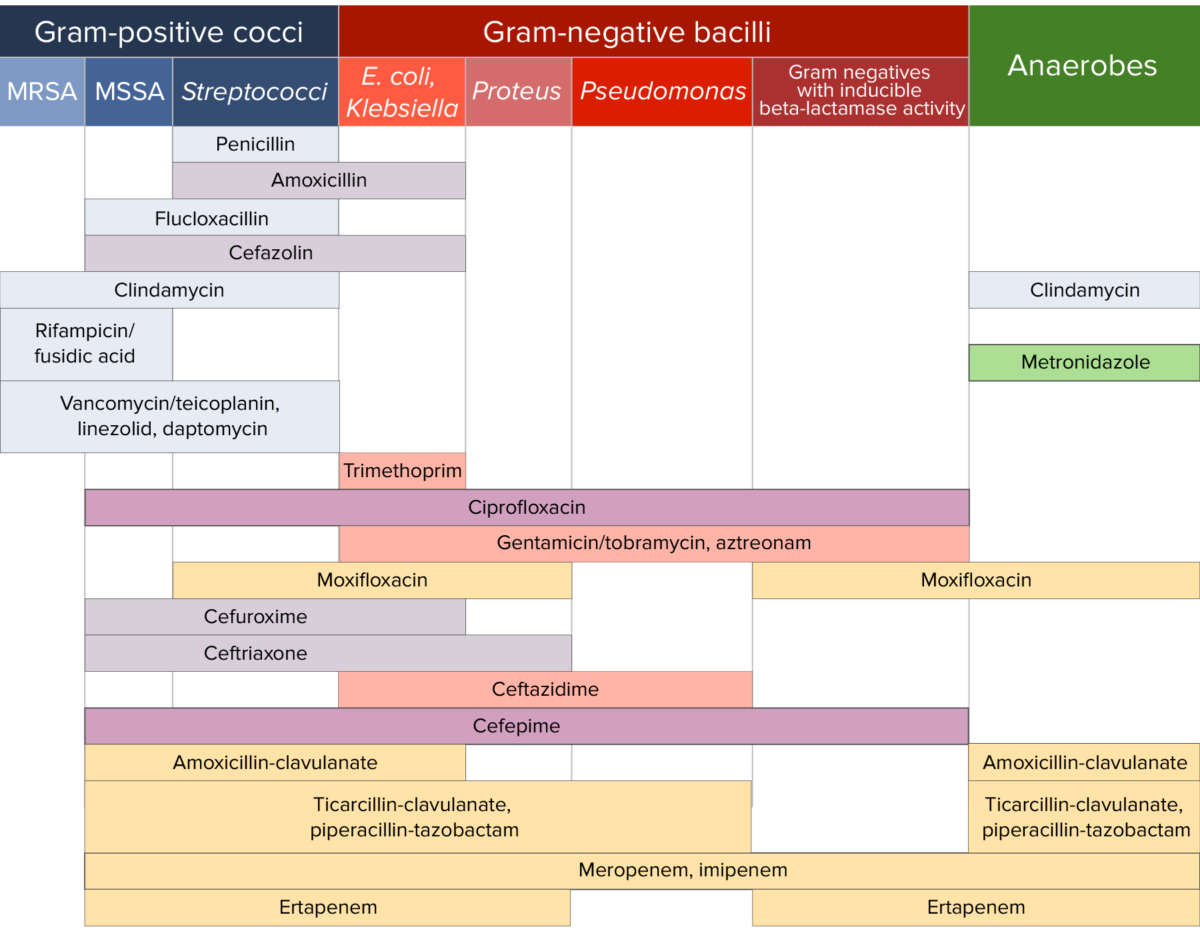
Antibiotic sensitivity:
Chart comparing the microbial coverage of different antibiotics for gram-positive cocci, gram-negative bacilli, and anaerobes.