Myelodysplastic syndromes (MDS) are a group of clonal neoplasms Neoplasms New abnormal growth of tissue. Malignant neoplasms show a greater degree of anaplasia and have the properties of invasion and metastasis, compared to benign neoplasms. Benign Bone Tumors with maturation defects characterized by dysplasia, cytopenia, and immature bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis precursors. Myelodysplastic syndromes can be idiopathic Idiopathic Dermatomyositis, or secondary to various injurious exposures such as cytotoxic Cytotoxic Parvovirus B19 chemotherapy Chemotherapy Osteosarcoma, ionizing radiation Radiation Emission or propagation of acoustic waves (sound), electromagnetic energy waves (such as light; radio waves; gamma rays; or x-rays), or a stream of subatomic particles (such as electrons; neutrons; protons; or alpha particles). Osteosarcoma, or environmental toxins. The median patient age is 70 years old. Presentation includes symptoms of anemia Anemia Anemia is a condition in which individuals have low Hb levels, which can arise from various causes. Anemia is accompanied by a reduced number of RBCs and may manifest with fatigue, shortness of breath, pallor, and weakness. Subtypes are classified by the size of RBCs, chronicity, and etiology. Anemia: Overview and Types ( fatigue Fatigue The state of weariness following a period of exertion, mental or physical, characterized by a decreased capacity for work and reduced efficiency to respond to stimuli. Fibromyalgia), neutropenia Neutropenia Neutrophils are an important component of the immune system and play a significant role in the eradication of infections. Low numbers of circulating neutrophils, referred to as neutropenia, predispose the body to recurrent infections or sepsis, though patients can also be asymptomatic. Neutropenia (infection), or thrombocytopenia Thrombocytopenia Thrombocytopenia occurs when the platelet count is < 150,000 per microliter. The normal range for platelets is usually 150,000-450,000/µL of whole blood. Thrombocytopenia can be a result of decreased production, increased destruction, or splenic sequestration of platelets. Patients are often asymptomatic until platelet counts are < 50,000/µL. Thrombocytopenia (bleeding). The diagnosis is based on bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis evaluation, which reveals cytopenia, dysplasia in at least 1 lineage, and blast cells in < 20% of marrow cellularity. Cytogenetic and molecular studies are required for classification, prognosis Prognosis A prediction of the probable outcome of a disease based on a individual's condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas, and therapy-related decisions. An increased cumulative risk of transformation Transformation Change brought about to an organism's genetic composition by unidirectional transfer (transfection; transduction, genetic; conjugation, genetic, etc.) and incorporation of foreign DNA into prokaryotic or eukaryotic cells by recombination of part or all of that DNA into the cell's genome. Bacteriology to AML AML Acute myeloid leukemia (AML) is a hematologic malignancy characterized by the uncontrolled proliferation of myeloid precursor cells. Seen predominantly in older adults, AML includes an accumulation of myeloblasts and a replacement of normal marrow by malignant cells, which leads to impaired hematopoiesis. Acute Myeloid Leukemia is present and varies depending on MDS subtype. Management includes supportive care, use of hematopoietic growth factors Hematopoietic growth factors Hematopoietic growth factors are a family of glycoproteins responsible for the proliferation and differentiation of hematopoietic progenitor cells in the bone marrow. Pharmacologic erythropoietin, thrombopoietin, granulocyte colony-stimulating factor (G-CSF), and granulocyte macrophage colony-stimulating factor (GM-CSF) are used in certain cases in which normal hematopoiesis is impaired owing to treatment (e.g., chemotherapy) or underlying disease (e.g., aplastic anemia). Hematopoietic Growth Factors, immunosuppressive therapy, and allogeneic hematopoietic cell transplantation.
Last updated: Dec 15, 2025
Myelodysplastic syndromes (MDS) are clonal, bone marrow diseases characterized by the presence of dysplastic, immature, bone marrow precursors and peripheral cytopenias. The World Health Organization (WHO) published an update in 2022 in which it renamed myelodysplastic syndromes “Myelodysplastic neoplasms (MDS)”.
The majority of MDS is attributed to chromosomal deletions and translocations, or gene Gene A category of nucleic acid sequences that function as units of heredity and which code for the basic instructions for the development, reproduction, and maintenance of organisms. Basic Terms of Genetics mutations.
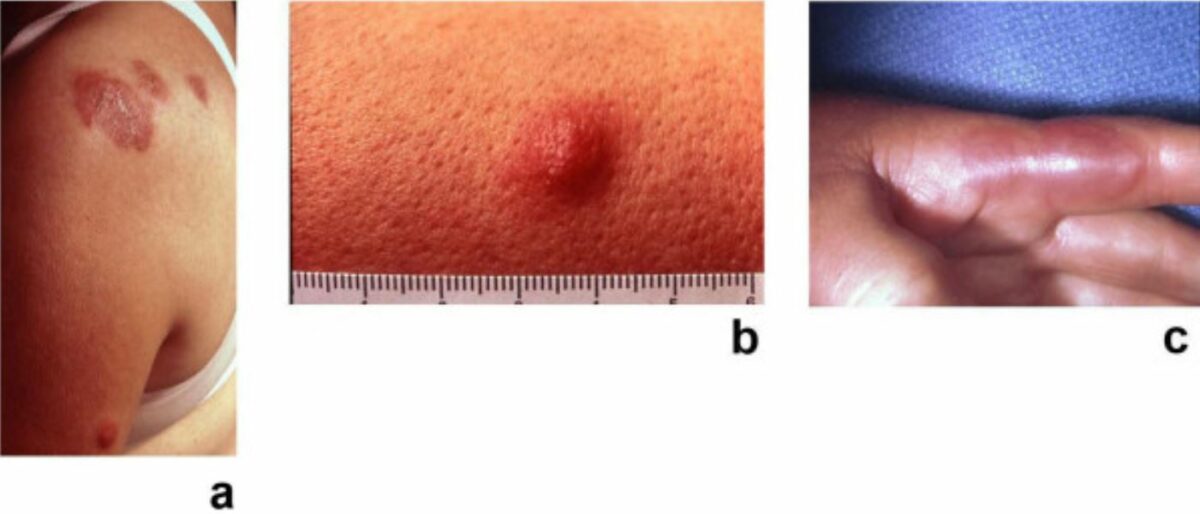
Sweet syndrome: painful, erythematous, pseudovascular plaques of acute febrile neutrophilic dermatosis
Image: “Sweet’s syndrome–a comprehensive review of an acute febrile neutrophilic dermatosis” by Cohen PR. License: CC BY 2.0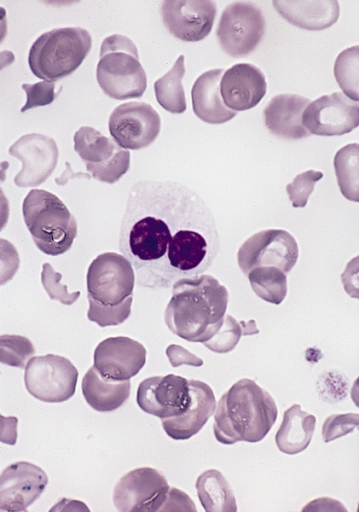
Peripheral smear in MDS showing a dysplastic neutrophil with bilobed nucleus and impaired granulation (pseudo–Pelger-Huët anomaly)
Image: “Hypogranular neutrophil with a pseudo-Pelger-Huet nucleus in MDS” by The Armed Forces Institute of Pathology (AFIP). License: Public Domain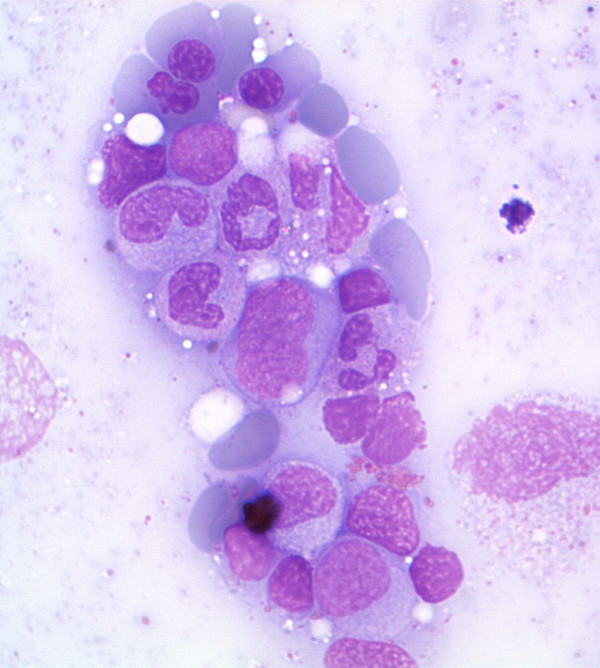
Bone marrow aspirate in MDS showing dysplastic erythroid cells and myeloid precursors
Image: “Antiretroviral activity of 5-azacytidine during treatment of a HTLV-1 positive myelodysplastic syndrome with autoimmune manifestations” by Diamantopoulos, P.T. et al. License: CC BY 2.0After MDS confirmation, patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship are categorized based on the 5th edition of the World Health Organization (WHO) Classification of Haematolymphoid Tumours, published in 2022.
Classification is based on:
Types: