Bacteriology is the branch of microbiology that deals with the morphology, structure, classification, and biochemistry of bacteria. The discipline of bacteriology arose during the 19th century from scientific attempts to prove the “germ theory of disease,” namely that diseases were caused by microscopic organisms invading host cells. Bacteria are prokaryotic Prokaryotic Prokaryotes are unicellular organisms that include 2 of the 3 domains of life: bacteria and archaea. Prokaryotic cells consist of a single cytoplasm-filled compartment enclosed by a cell membrane and cell wall. Cell Types: Eukaryotic versus Prokaryotic single-celled microorganisms that are metabolically active and divide by binary fission Binary fission Cell Types: Eukaryotic versus Prokaryotic. Some of these organisms play a significant role in the pathogenesis of diseases. Management of bacterial disease is generally with antibiotics; however, the choice of antibiotics may vary depending on the bacterial structure and metabolism.
Last updated: Dec 15, 2025
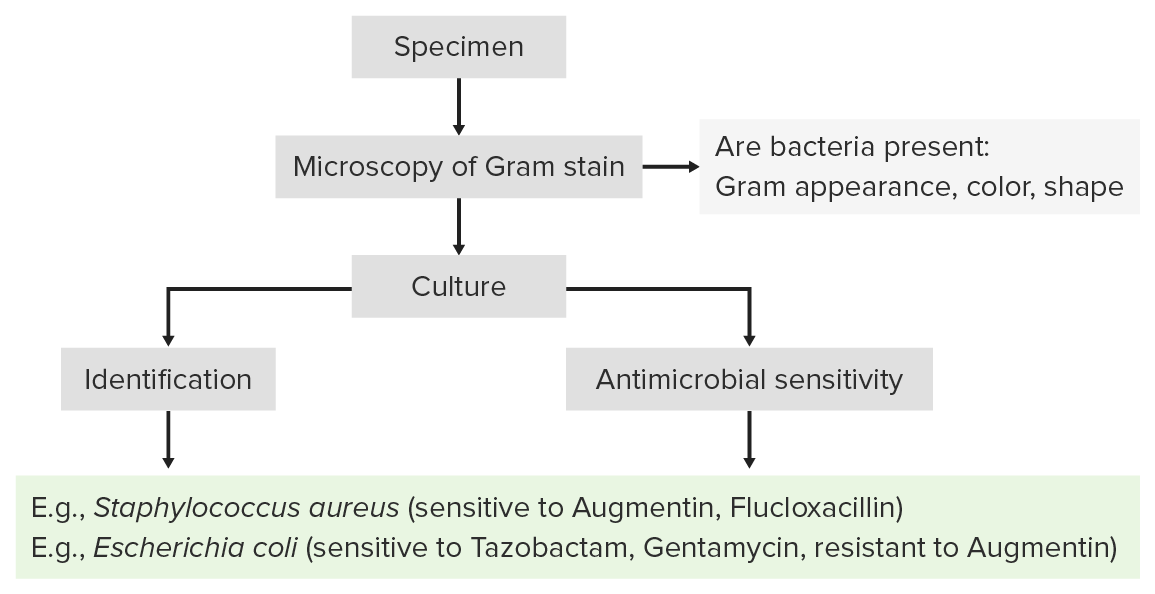
Process of laboratory identification:
Identification of bacterial pathogens follows a stepwise process that usually begins with Gram staining and is followed by growth in isolated culture.
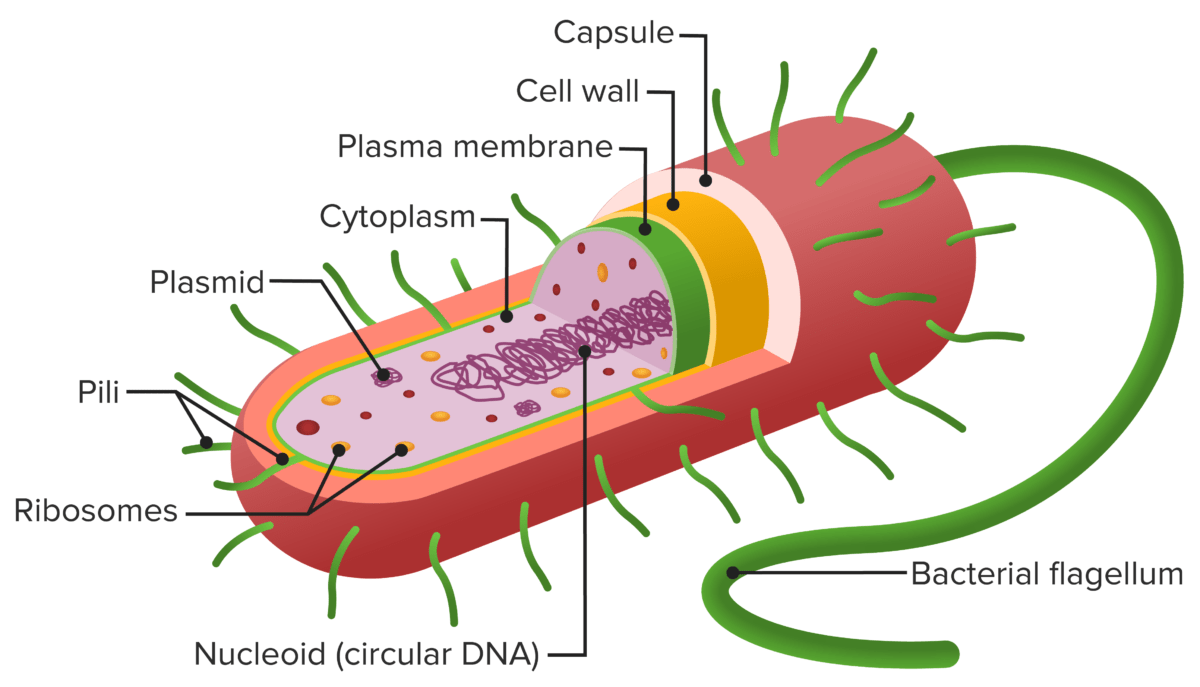
Structure of a prokaryote cell:
The cell envelope comprises a plasma membrane (green layer) and a thick, peptidoglycan-containing cell wall (yellow layer). No outer lipid membrane is present (as seen in gram-negative bacteria). The capsule (red layer) is distinct from the cell envelope.
| Structure | Chemical composition | Function |
|---|---|---|
| Appendages | ||
| Flagellum | Protein | Motility Motility The motor activity of the gastrointestinal tract. Gastrointestinal Motility |
| Pili Pili Filamentous or elongated proteinaceous structures which extend from the cell surface in gram-negative bacteria that contain certain types of conjugative plasmid. These pili are the organs associated with genetic transfer and have essential roles in conjugation. Normally, only one or a few pili occur on a given donor cell. This preferred use of ‘pili’ refers to the sexual appendage, to be distinguished from bacterial fimbriae, also known as common pili, which are usually concerned with adhesion. Salmonella/ fimbriae Fimbriae Thin, hairlike appendages, 1 to 20 microns in length and often occurring in large numbers, present on the cells of gram-negative bacteria, particularly enterobacteriaceae and Neisseria. Unlike flagella, they do not possess motility, but being protein (pilin) in nature, they possess antigenic and hemagglutinating properties. They are of medical importance because some fimbriae mediate the attachment of bacteria to cells via adhesins. Bacterial fimbriae refer to common pili, to be distinguished from the preferred use of ‘pili’. Escherichia coli | Glycoprotein | Adherence to cell surface |
| Specialized structures | ||
| Spore Spore The reproductive elements of lower organisms, such as bacteria; fungi; and cryptogamic plants. Microsporidia/Microsporidiosis |
|
|
| Cell envelope Envelope Bilayer lipid membrane acquired by viral particles during viral morphogenesis. Although the lipids of the viral envelope are host derived, various virus-encoded integral membrane proteins, i.e. Viral envelope proteins are incorporated there. Virology | ||
| Capsule Capsule An envelope of loose gel surrounding a bacterial cell which is associated with the virulence of pathogenic bacteria. Some capsules have a well-defined border, whereas others form a slime layer that trails off into the medium. Most capsules consist of relatively simple polysaccharides but there are some bacteria whose capsules are made of polypeptides. Bacteroides | Organized polysaccharide layer | Protects against phagocytosis Phagocytosis The engulfing and degradation of microorganisms; other cells that are dead, dying, or pathogenic; and foreign particles by phagocytic cells (phagocytes). Innate Immunity: Phagocytes and Antigen Presentation |
| Slime layer | Loose network of polysaccharides Polysaccharides Basics of Carbohydrates | Mediates adherence to surfaces |
| Outer membrane |
|
|
| Periplasm | Peptidoglycan Peptidoglycan Penicillins in middle | Accumulate components exiting gram- cells |
| Cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic | Peptidoglycan Peptidoglycan Penicillins in sugar backbone | Net-like structure gives rigid support. |
| Cytoplasmic membrane | Phospholipid bilayer sac |
|
Gram staining is a technique named after the bacteriologist Hans Christian Joachim Gram, and is used to differentiate between groups of bacteria based on the differences in the constituents of their cell walls.
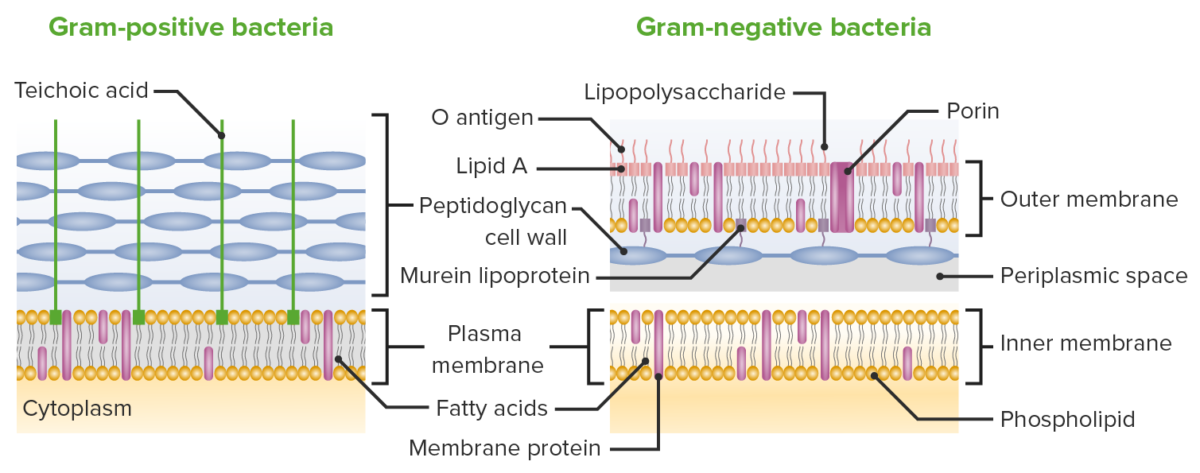
Differences between the cell membranes of gram-positive and gram-negative bacteria:
While both gram-positive and gram-negative bacterial cell walls contain peptidoglycan layers, the layer in gram-negative bacteria is much thinner. Gram-negative bacteria make up for this deficit by having another membrane layer outside the peptidoglycan layer.
Gram staining helps distinguish between gram-positive Gram-Positive Penicillins and gram-negative bacteria by staining cells red or violet.
Process of Gram staining:
Staining of gram-positive Gram-Positive Penicillins bacteria:
Staining of gram-negative bacteria:
Staining plays important role in diagnostics/pathology:
| Morphology | Arrangements |
|---|---|
| Cocci |
|
| Bacilli Bacilli Shigella |
|
| Spirilla |
|
| Spirochetes Spirochetes An order of slender, flexuous, helically coiled bacteria, with one or more complete turns in the helix. Treponema |
|
| Genus Selenomonas | Cylinders curved in a plane |
| Genus Haloquadratum |
|
| Vibrions |
|
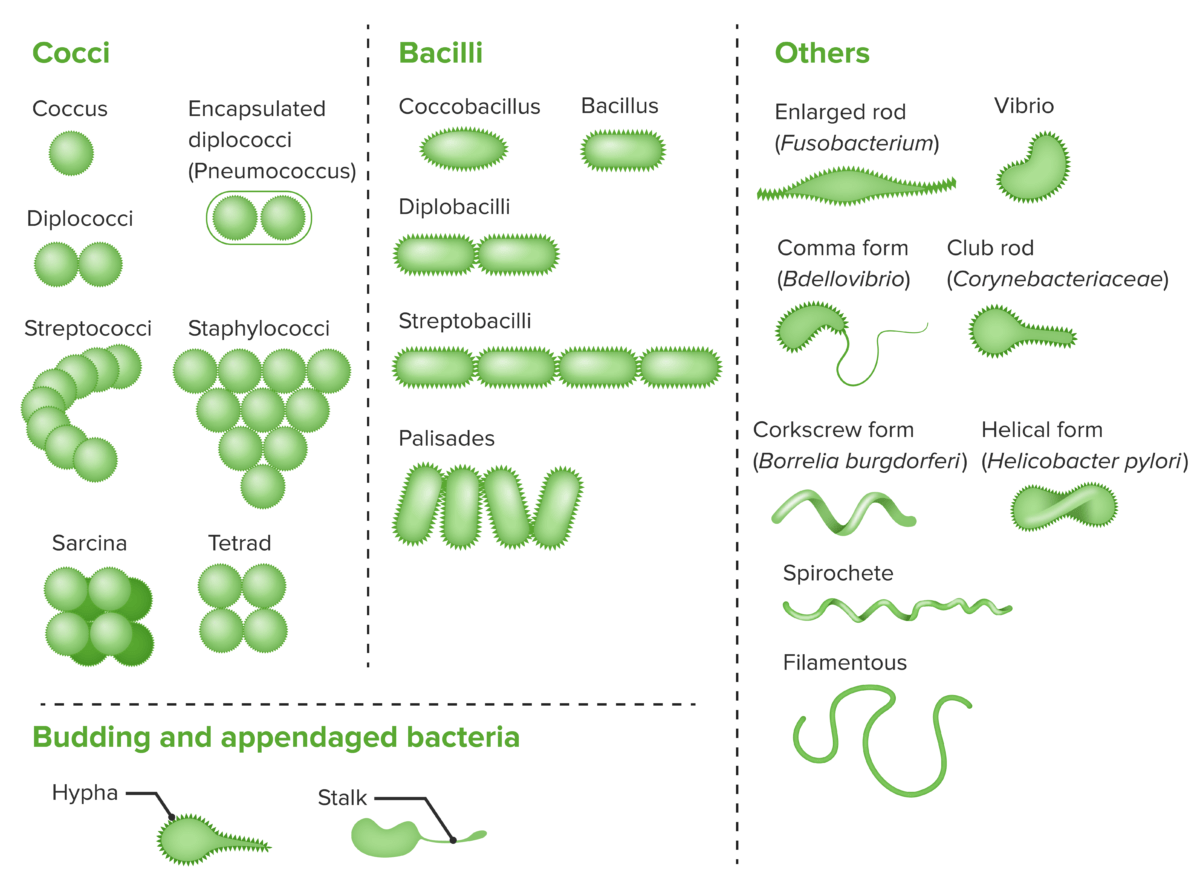
Bacterial cells with various morphologies and arrangements:
Bacteria exist in a wide variety of morphologies and arrangements. Cocci and bacilli in pairs and clusters are among the most common.
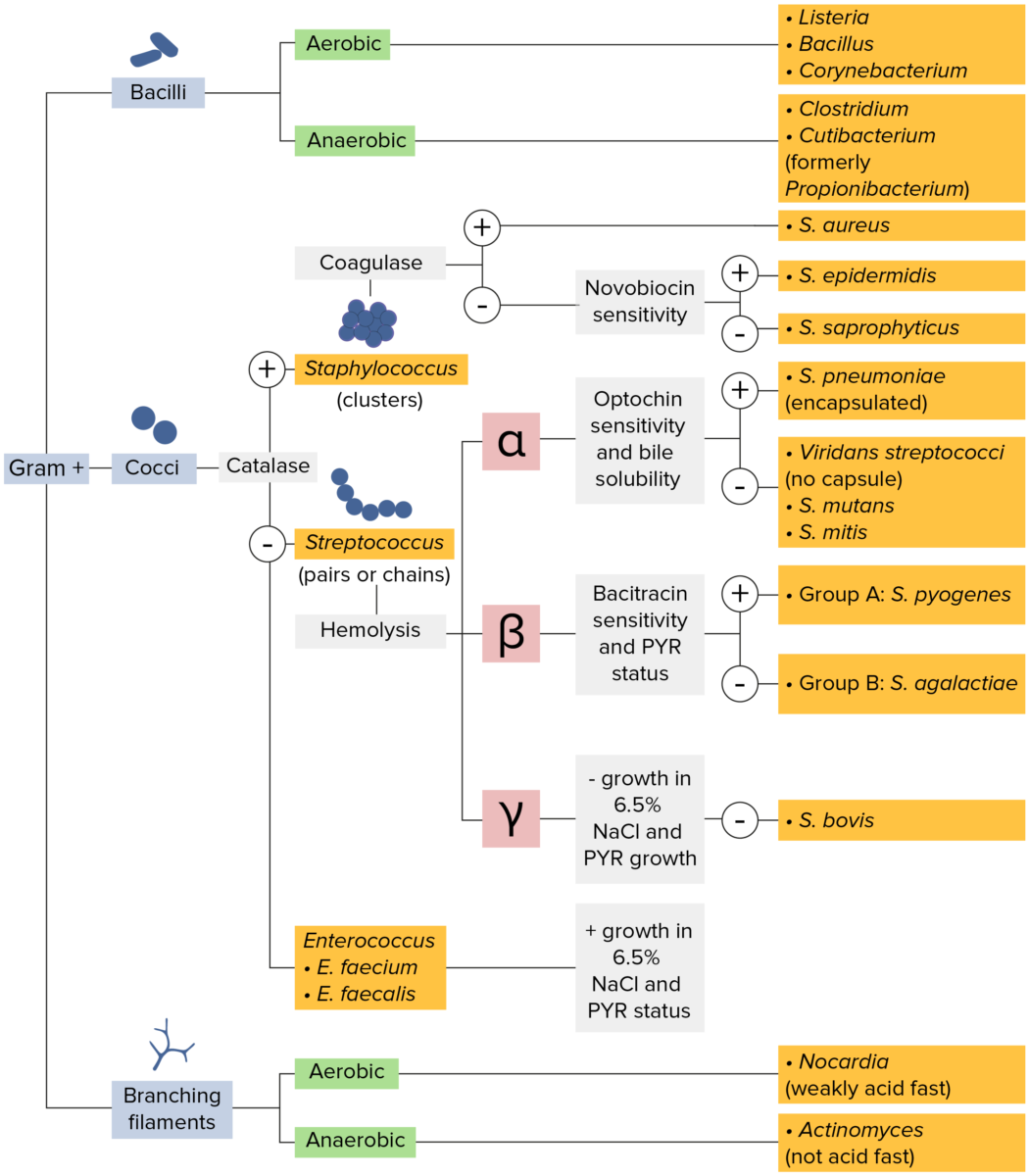
Gram-positive bacteria:
Most bacteria can be classified according to a lab procedure called Gram staining.
Bacteria with cell walls that have a thick layer of peptidoglycan retain the crystal violet stain utilized in Gram staining but are not affected by the safranin counterstain. These bacteria appear as purple-blue on the stain, indicating that they are gram positive. The bacteria can be further classified according to morphology (branching filaments, bacilli, and cocci in clusters or chains) and their ability to grow in the presence of oxygen (aerobic versus anaerobic). The cocci can also be further identified. Staphylococci can be narrowed down on the basis of the presence of the enzyme coagulase and on their sensitivity to the antibiotic novobiocin. Streptococci are grown on blood agar and classified on the basis of which form of hemolysis they employ (α, β, or γ). Streptococci are further narrowed on the basis of their response to the pyrrolidonyl-β-naphthylamide (PYR) test, their sensitivity to specific antimicrobials (optochin and bacitracin), and their ability to grow on sodium chloride (NaCl) media.
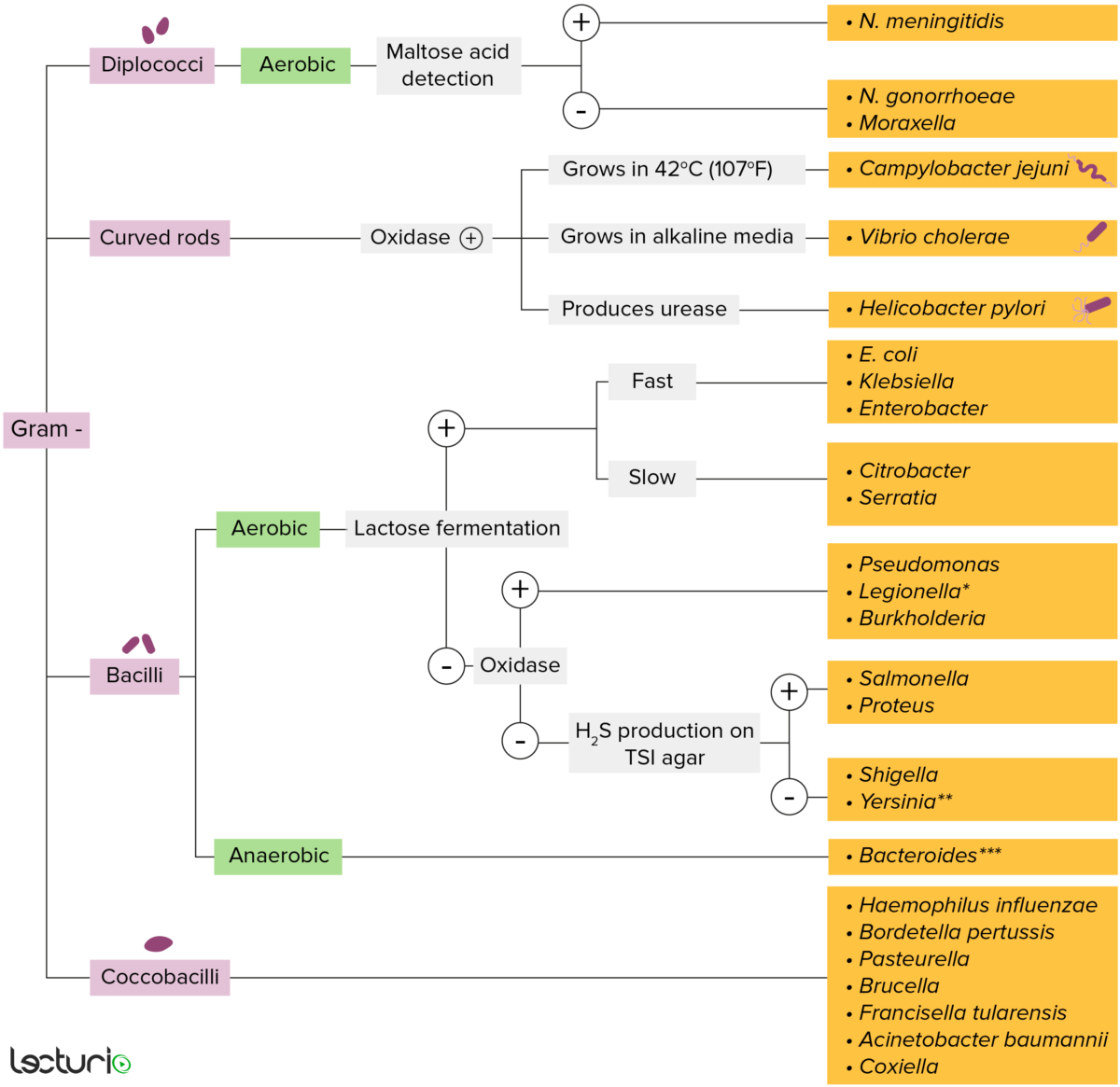
Gram-negative bacteria:
Most bacteria can be classified based on a lab procedure called Gram staining.
Bacterial cell walls having a thin layer of peptidoglycan do not retain the crystal violet stain used for Gram staining. However, gram-negative bacteria retain the safranin counterstain and appear pinkish-red. These bacteria can be further classified according to morphology (diplococci, curved rods, bacilli, and coccobacilli) and their ability to grow in the presence of oxygen (aerobic versus anaerobic). Gram-negative bacteria can be accurately identified by culturing on specific media (triple sugar iron (TSI) agar), where their enzymes can be identified (urease, oxidase) and their ability to ferment lactose can be determined.
* Stains poorly on Gram stain
** Pleomorphic rod/coccobacillus
*** Require special transport media
Bacteria are heterotrophic organisms that need organic substances to survive.
Classified based on oxygen requirements:
Bacteria can exchange genetic material:
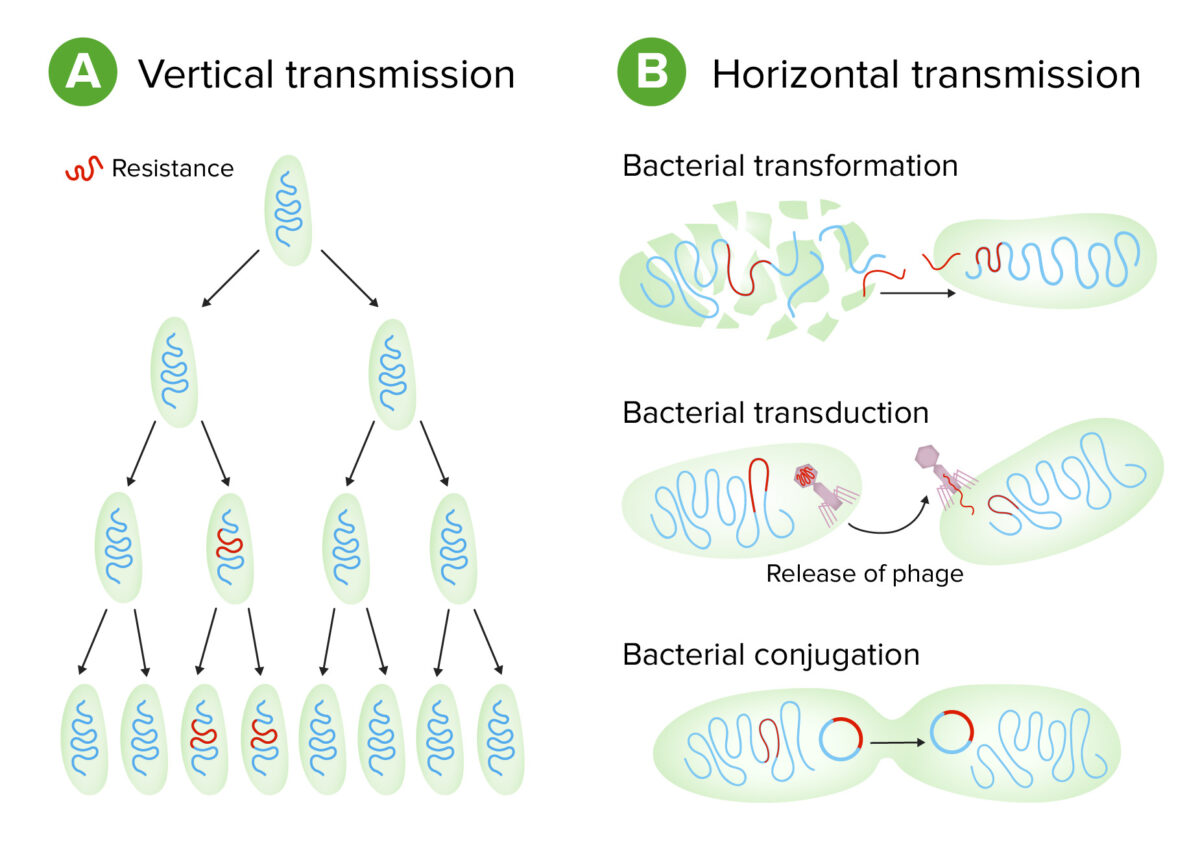
Schematic of types of genetic transmission in bacteria
Image by Lecturio.Virulence Virulence The degree of pathogenicity within a group or species of microorganisms or viruses as indicated by case fatality rates and/or the ability of the organism to invade the tissues of the host. The pathogenic capacity of an organism is determined by its virulence factors. Proteus is the degree to which an organism can infect a host and cause disease. Virulence factors Virulence factors Those components of an organism that determine its capacity to cause disease but are not required for its viability per se. Two classes have been characterized: toxins, biological and surface adhesion molecules that affect the ability of the microorganism to invade and colonize a host. Haemophilus are molecules that assist the bacterium in infecting the host and can be either secretory, membrane associated, or cytosolic in nature.
| Mechanism | Virulence factors Virulence factors Those components of an organism that determine its capacity to cause disease but are not required for its viability per se. Two classes have been characterized: toxins, biological and surface adhesion molecules that affect the ability of the microorganism to invade and colonize a host. Haemophilus | Function |
|---|---|---|
| Colonization |
|
|
| Avoiding the immune system Immune system The body’s defense mechanism against foreign organisms or substances and deviant native cells. It includes the humoral immune response and the cell-mediated response and consists of a complex of interrelated cellular, molecular, and genetic components. Primary Lymphatic Organs |
|
Creates physical barrier blocking opsonization and phagocytosis Phagocytosis The engulfing and degradation of microorganisms; other cells that are dead, dying, or pathogenic; and foreign particles by phagocytic cells (phagocytes). Innate Immunity: Phagocytes and Antigen Presentation |
| Bacterial nutrition | Siderophores Siderophores Low-molecular-weight compounds produced by microorganisms that aid in the transport and sequestration of ferric iron. Klebsiella |
|
| Antigenic variation |
|
Camouflage of surface molecular markers that allow evasion of the immune system Immune system The body’s defense mechanism against foreign organisms or substances and deviant native cells. It includes the humoral immune response and the cell-mediated response and consists of a complex of interrelated cellular, molecular, and genetic components. Primary Lymphatic Organs |
| Intracellular survival |
|
Prevents intracellular destruction of the bacteria |
| Type III secretion Secretion Coagulation Studies system | Injectisome | Allows bacteria to inject toxins into host cells |
| Inflammatory response |
|
|