Achalasia is an esophageal motility disorder that is due to degeneration of the myenteric plexus; it results in failure of the lower esophageal sphincter to relax and a lack of normal esophageal peristalsis. Patients typically present with dysphagia to solids and liquids, along with regurgitation. Diagnosis is established by high-resolution manometry. Upper endoscopy is performed to rule out malignancy as a cause. Barium swallow study helps evaluate the esophageal morphology. Management options include pneumatic balloon dilation, surgical myotomy, and botulinum toxin injection. Choice of treatment is dependent on the type of achalasia and surgical risk. Medications are available for those who fail initial intervention, but they provide the least benefit.
Last updated: May 26, 2025
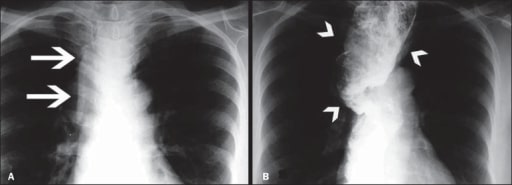
Image of megaesophagus (loss of esophageal motility and diffuse esophageal dilation)
A: Posteroanterior X-ray showing widening of the superior mediastinum (arrows)
B: Barium swallow study demonstrating a dilated and tortuous esophagus (arrowheads)
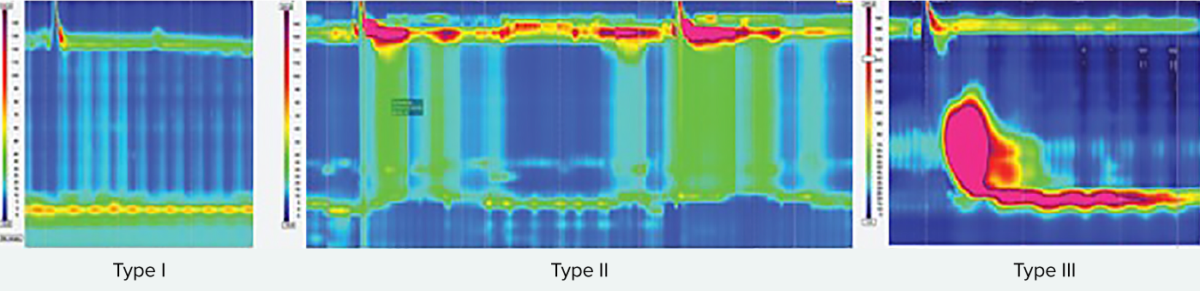
Types of achalasia
All types exhibit impaired esophagogastric junction relaxation.
Type I: On swallow, the upper esophageal sphincter relaxed, but is followed by 100% failed contractions and no esophageal pressurization (absent peristalsis).
Type II has panesophageal pressurization in at least 20% of swallows.
Type III is defined by the presence of preserved fragments of distal peristalsis or premature contractions for at least 20% of the swallows (spastic).
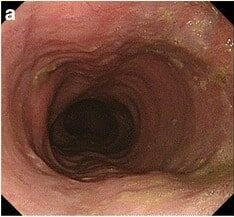
Esophagogastroduodenoscopy showcasing a dilated esophagus with food residue in a patient presenting with achalasia
Image: “Gastrointestinal endoscopy findings” by US National Library of Medicine. License: CC BY 4.0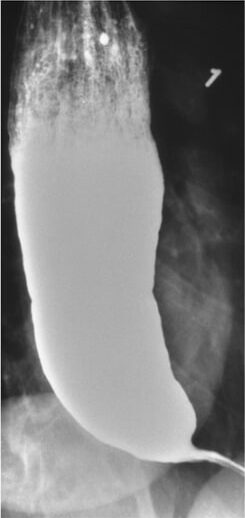
Achalasia in a barium swallow study: Narrowing is seen at the distal esophagus (“bird’s beak”) due to inadequate relaxation of the lower esophageal sphincter.
Image: “Barium swallow” by Department of Internal Medicine, Nashville, TN, USA. License: CC BY 4.0