Mitral valve Mitral valve The valve between the left atrium and left ventricle of the heart. Heart: Anatomy prolapse (MVP) is the most common cardiac valvular defect, and is characterized by bulging of the mitral valve Mitral valve The valve between the left atrium and left ventricle of the heart. Heart: Anatomy (MV) cusps into the left atrium (LA) during systole Systole Period of contraction of the heart, especially of the heart ventricles. Cardiac Cycle. Mitral valve Mitral valve The valve between the left atrium and left ventricle of the heart. Heart: Anatomy prolapse is most commonly due to idiopathic Idiopathic Dermatomyositis myxomatous degeneration. Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship are typically asymptomatic. Auscultation generally reveals a mid-to-late systolic click, followed by a possible regurgitation Regurgitation Gastroesophageal Reflux Disease (GERD) murmur. The diagnosis is confirmed by an echocardiogram Echocardiogram Transposition of the Great Arteries. Treatment is generally not required for asymptomatic patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship.
Last updated: May 12, 2025
Mitral valve Mitral valve The valve between the left atrium and left ventricle of the heart. Heart: Anatomy prolapse (MVP) is the bulging of the mitral valve Mitral valve The valve between the left atrium and left ventricle of the heart. Heart: Anatomy (MV) leaflets into the atrium by ≥ 2 mm above the plane of the MV annulus during ventricular systole Ventricular systole Cardiac Cycle:
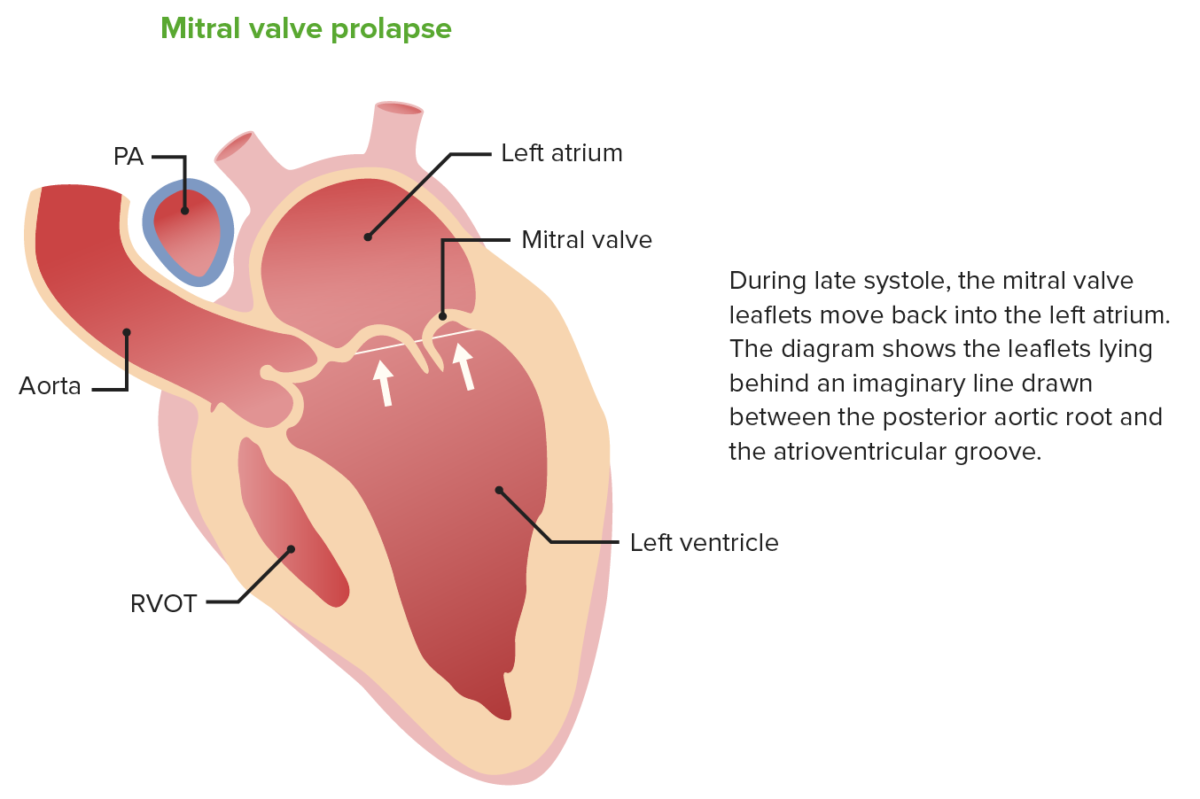
Billowing of the mitral valve leaflets into the left atrium during systole in mitral valve prolapse
Image by Lecturio.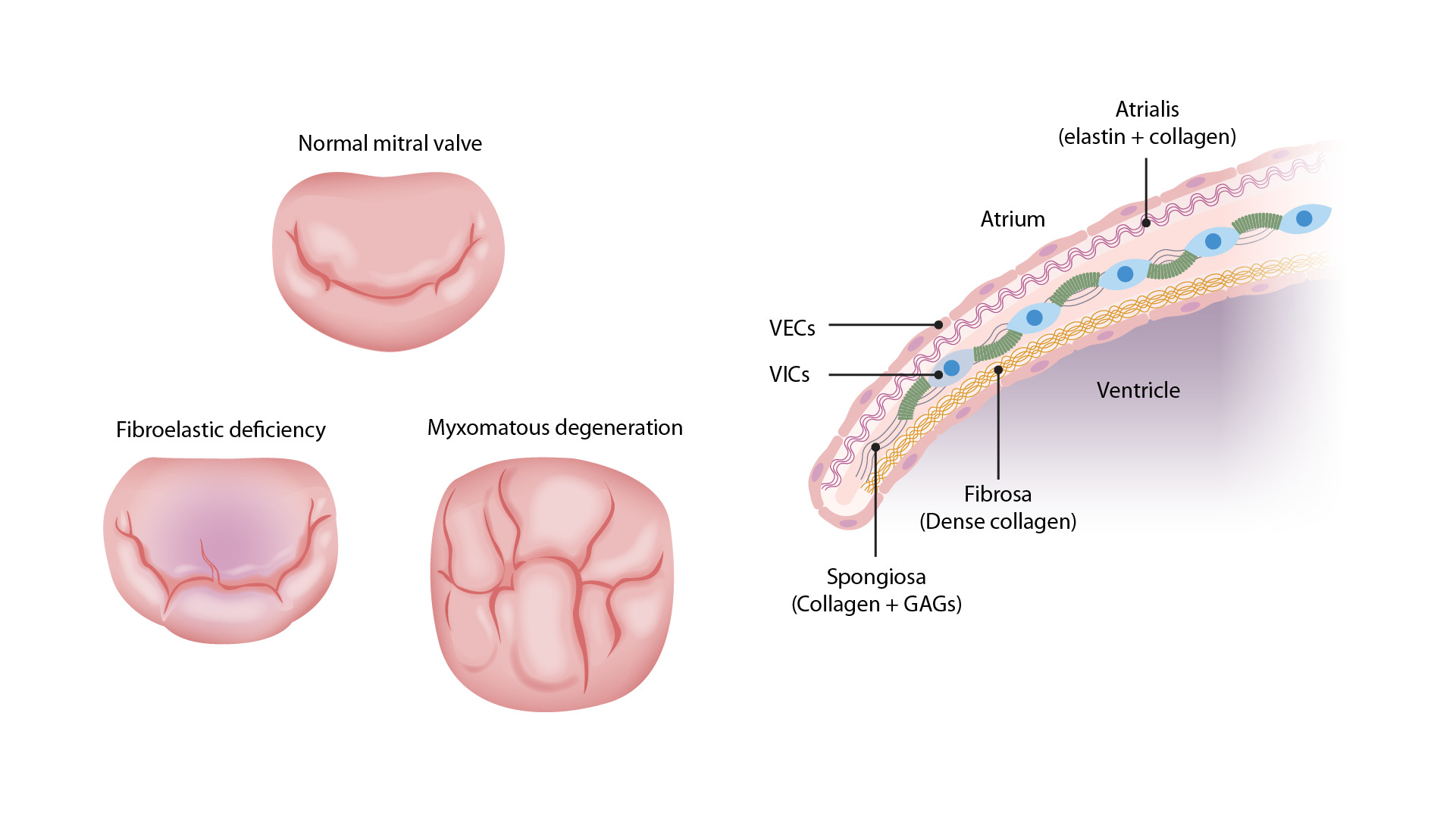
Left: Images comparing the different pathologies seen in MVP. The top valve is a normal MV with the anterior (upper) and posterior (lower) leaflets. The bottom right valve shows thickened, redundant leaflet tissue of myxomatous degeneration, which can billow into the atrium during systole. The bottom left valve has the thinned leaflets of fibroelastic deficiency. The chordae tendinae are also thin and can easily rupture, allowing the leaflet to flail into the atrium.
Right: Image demonstrating a normal MV. There are 3 layers: the atrialis, the spongiosa, and the fibrosa. Myxomatous degeneration in MVP requires proliferation of the spongiosa layer with increased GAGs. There will also be altered collagen composition and elastin fragmentation, which can disrupt the valvular endothelial cells (VECs), which can become sites for endocarditis and thrombus formation.
VICs = valvular interstitial cells
Image by Lecturio.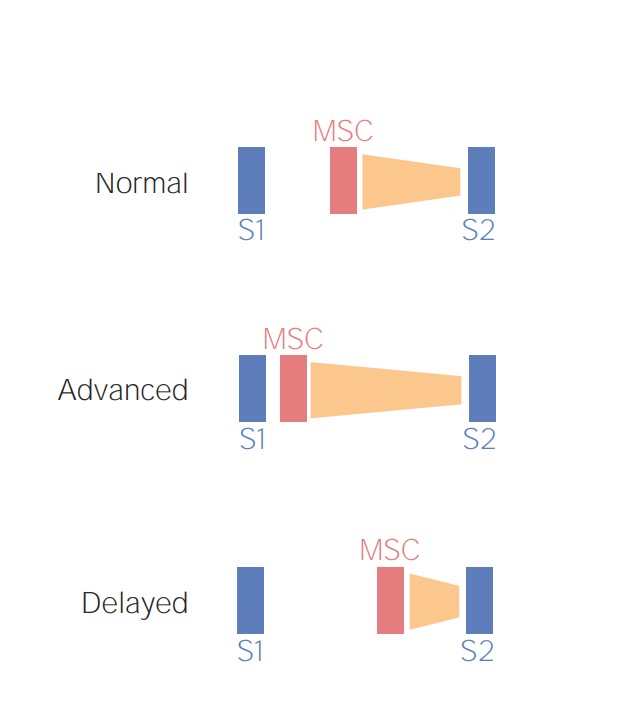
Schematic diagram depicting the preload-dependent changes in the mid-systolic click (MSC) and late-systolic murmur (orange). Increased venous return (e.g., squatting), causes a delay in the MSC-murmur complex while a reduced venous return (e.g., standing) would bring the MSC-murmur complex closer to S1.
Image by Lecturio.Audio:
This audio clip is an example of the mid-systolic click heard in mitral valve Mitral valve The valve between the left atrium and left ventricle of the heart. Heart: Anatomy prolapse. This is a crisp sound occurring between S1 S1 Heart Sounds and S2 S2 Heart Sounds. No murmur is noted.
Audio:
This audio clip demonstrates a mid-systolic click with a late systolic murmur due to mitral valve Mitral valve The valve between the left atrium and left ventricle of the heart. Heart: Anatomy prolapse. There is a crisp click, followed by a high-pitched, blowing murmur.
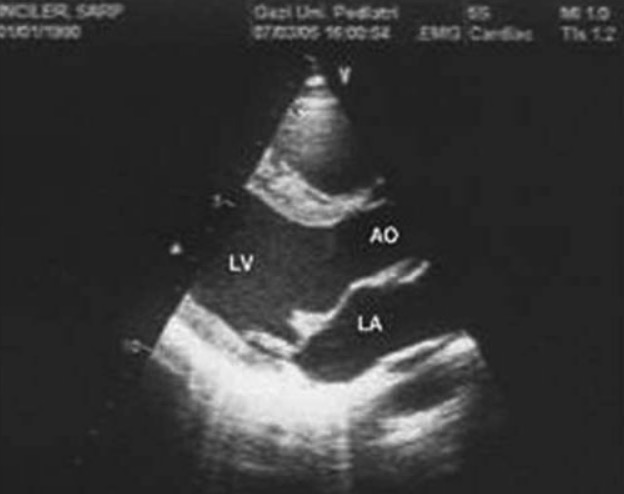
Mitral valve prolapse in cross-sectional echocardiographic examination
AO: aorta, LA: left atrium, LV: left ventricle
Image: “Mitral valve prolapse” by Kecioren Training and Research Hospital, Ankara, Turkey. License: CC BY 2.5, edited by Lecturio.
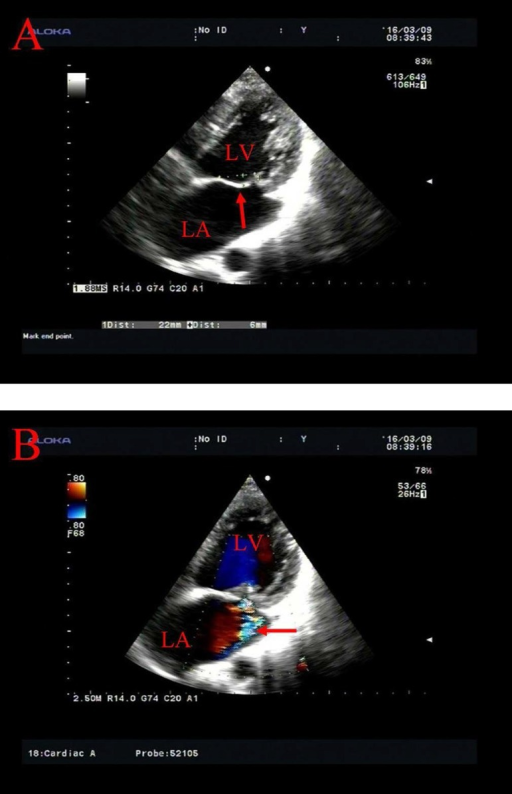
Transthoracic echocardiography of MV prolapse.
A: prolapse of the anterior MV leaflet behind the annular plane (arrow)
B: mitral insufficiency with a regurgitant flow along the posterior wall of the LA (arrow)
LA: left atrium; LV: left ventricle