Ovotesticular disorder of sexual development (ODSD), formerly known as true hermaphroditism, is characterized by the presence of an ovotesticular gonad that contains both ovarian and testicular elements. Individuals are usually born with ambiguous genitalia Ambiguous Genitalia Primary Amenorrhea, but the diagnosis is rarely confirmed before puberty Puberty Puberty is a complex series of physical, psychosocial, and cognitive transitions usually experienced by adolescents (11-19 years of age). Puberty is marked by a growth in stature and the development of secondary sexual characteristics, achievement of fertility, and changes in most body systems. Puberty. The most common karyotype Karyotype The full set of chromosomes presented as a systematized array of metaphase chromosomes from a photomicrograph of a single cell nucleus arranged in pairs in descending order of size and according to the position of the centromere. Congenital Malformations of the Female Reproductive System is 46,XX, and less often, 46,XY can be identified. Gonadal biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma with histologic examination confirms the diagnosis. Management needs to consider the individual’s preferences wherever possible. Treatment options range from hormone replacement to surgical removal of part of the ovotestis gonad to improve fertility and to decrease the risk of malignancies.
Last updated: Jan 24, 2025
Ovotesticular disorder of sexual development (ODSD) is characterized by the presence of gonads Gonads The gamete-producing glands, ovary or testis. Hormones: Overview and Types that contain ovarian tissue with primordial follicles and testicular tissue with seminiferous tubules Seminiferous Tubules The convoluted tubules in the testis where sperm are produced (spermatogenesis) and conveyed to the rete testis. Spermatogenic tubules are composed of developing germ cells and the supporting sertoli cells. Testicles: Anatomy in the same individual.
An affected person may have:
The type of internal genitalia present depends on the adjacent gonad:
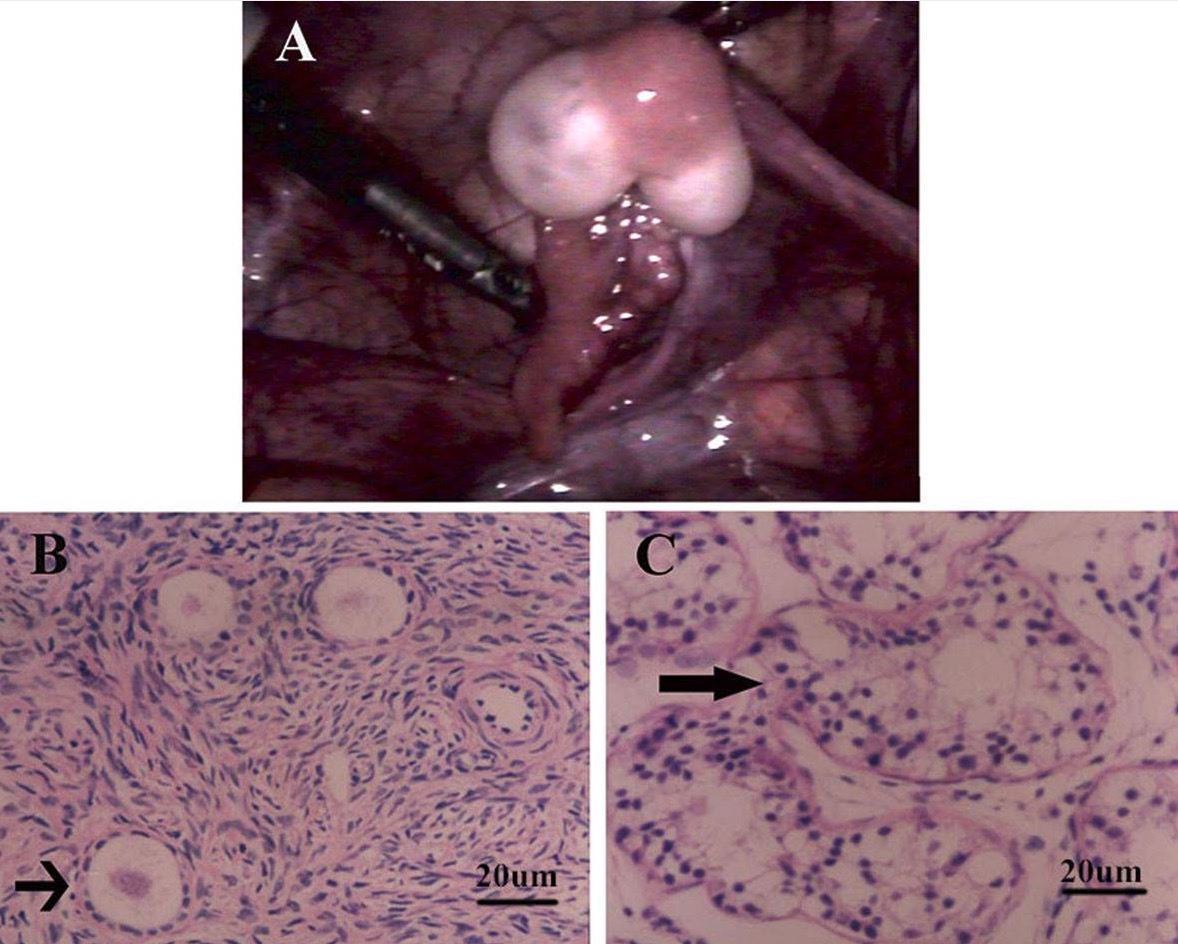
Ovotestis gonads
A: Gross appearance of a bilobed ovotestis with a fallopian tube: The tan-yellow tissue in the center of the ovotestis revealed ovarian tissue and the white tissue at each pole showed testicular tissue on histologic examination.
B: Microscopic examination of the tan-yellow tissue showed typical ovarian stroma with primordial and primary follicles (arrow), and examination of the white tissue.
C: Image shows unremarkable testicular tissue with well-developed seminiferous tubules (arrow), with no definite spermatids.
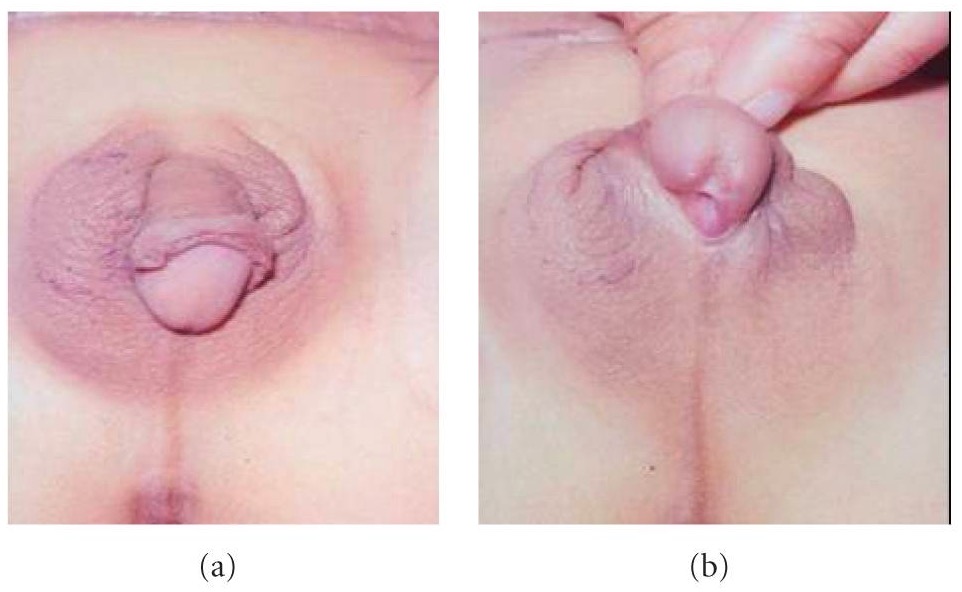
External genitalia in disorder of sexual development:
An individual with ODSD with a well-developed phallus and right scrotal ovotestis gonad (a), and penoscrotal hypospadias (b)