The pH of the blood is tightly regulated within the range 7.35–7.45 to ensure proper physiologic functions. Large amounts of acid are generated each day through normal processes (aerobic/anaerobic respiration Respiration The act of breathing with the lungs, consisting of inhalation, or the taking into the lungs of the ambient air, and of exhalation, or the expelling of the modified air which contains more carbon dioxide than the air taken in. Nose Anatomy (External & Internal) and dietary intake), and these are efficiently managed and eliminated by buffers in the blood, the respiratory system, and the renal system. When these regulatory systems are disturbed, acid–base balance disorders occur, including respiratory acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis, respiratory alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis, metabolic acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis, and metabolic alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis.
Last updated: Dec 15, 2025
pH is the quantitative measurement of the acidity or basicity of a solution.
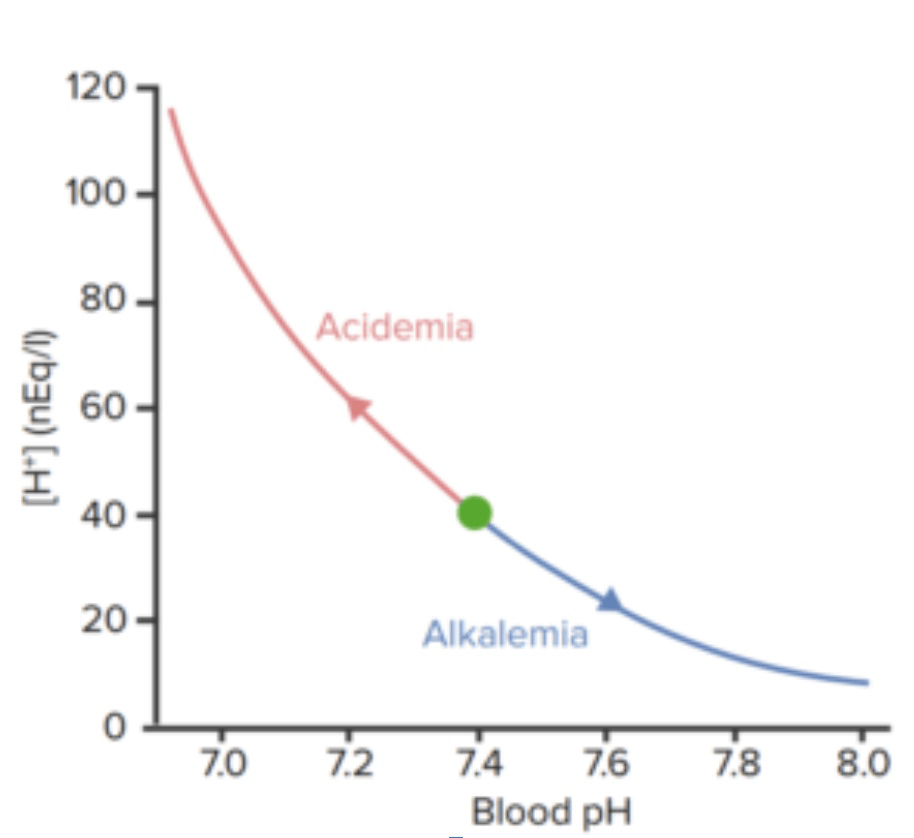
Relation between blood pH and concentration of hydrogen ions
Image by Lecturio.| Compartment | pH |
|---|---|
| Gastric secretions Gastric secretions Gastrointestinal Secretions (under conditions of maximal acidity) | 0.7 |
| Lysosome | 5.5 |
| Chromaffin granule | 5.5 |
| Neutral H2O at 37°C | 6.81 |
| Cytosol Cytosol A cell’s cytoskeleton is a network of intracellular protein fibers that provides structural support, anchors organelles, and aids intra- and extracellular movement. The Cell: Cytosol and Cytoskeleton of a typical cell | 6.0–7.4 |
| CSF | 7.3 |
| Arterial blood plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products | 7.35–7.45 |
| Mitochondrial inner matrix | 7.5 |
| Pancreatic secretions Pancreatic Secretions Gastrointestinal Secretions | 8.1 |
The relative concentrations of acids and bases in the blood determine its pH. Buffers provide a short-term solution for disturbances in this balance before the lungs Lungs Lungs are the main organs of the respiratory system. Lungs are paired viscera located in the thoracic cavity and are composed of spongy tissue. The primary function of the lungs is to oxygenate blood and eliminate CO2. Lungs: Anatomy and kidneys Kidneys The kidneys are a pair of bean-shaped organs located retroperitoneally against the posterior wall of the abdomen on either side of the spine. As part of the urinary tract, the kidneys are responsible for blood filtration and excretion of water-soluble waste in the urine. Kidneys: Anatomy can act definitively to restore balance.
Acids are compounds that can donate protons (H+) or accept electrons.
Bases are compounds that can accept protons (H+) or donate electrons.
Buffers are substances that consume or releases hydrogen ions (H+) to stabilize the pH.
| Normal ABG | Acidic ABG | Alkalotic ABG | |
|---|---|---|---|
| HCO3– | 24 mEq/L | 26 mEq/L | 22 mEq/L |
| PaCO2 | 40 mm Hg | 60 mm Hg | 20 mm Hg |
| pH | 7.40 | 7.26 | 7.66 |
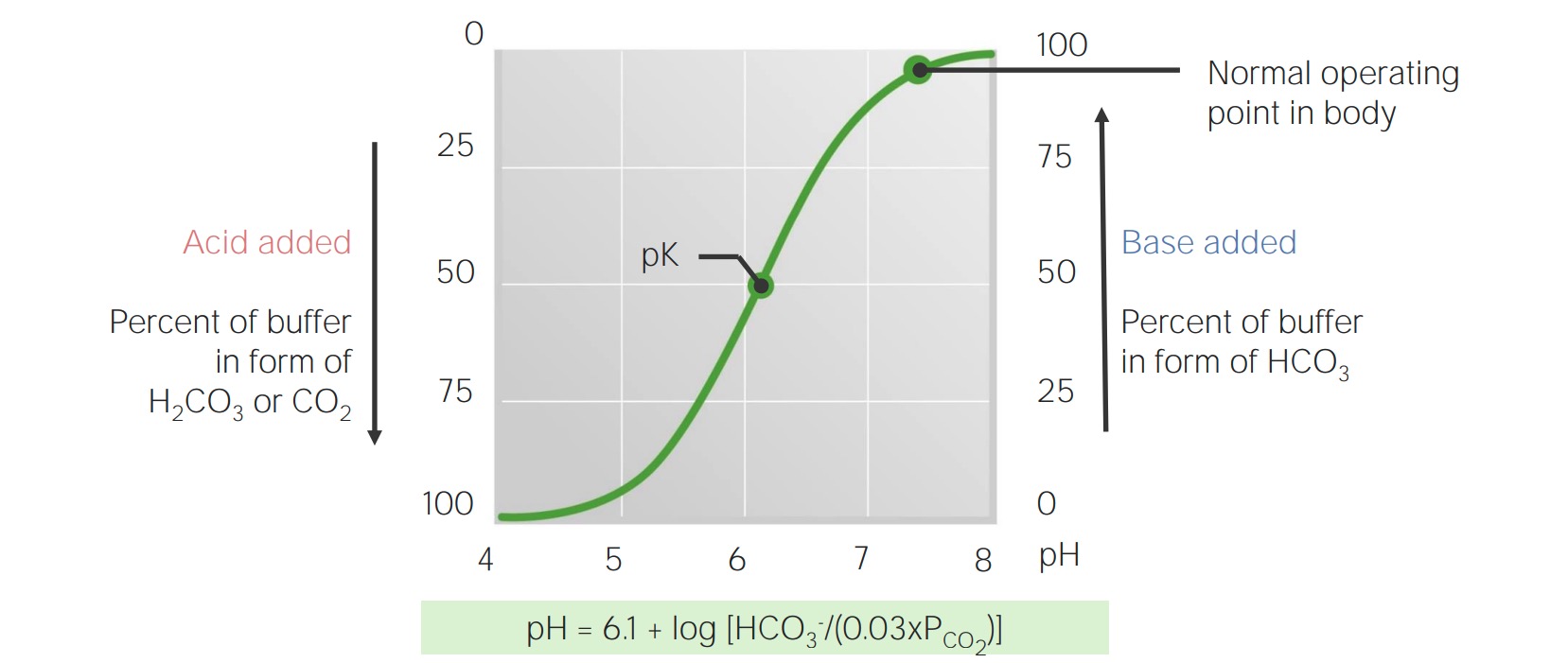
Titration curve for bicarbonate in the blood
Image by Lecturio.The body produces approximately 15,000 mmol of volatile and 70 mmol of nonvolatile acids daily. The lungs Lungs Lungs are the main organs of the respiratory system. Lungs are paired viscera located in the thoracic cavity and are composed of spongy tissue. The primary function of the lungs is to oxygenate blood and eliminate CO2. Lungs: Anatomy and kidneys Kidneys The kidneys are a pair of bean-shaped organs located retroperitoneally against the posterior wall of the abdomen on either side of the spine. As part of the urinary tract, the kidneys are responsible for blood filtration and excretion of water-soluble waste in the urine. Kidneys: Anatomy work in concert to eliminate this daily acid load, which prevents the buffering capability of the blood from being overwhelmed and allows it to maintain a normal pH.
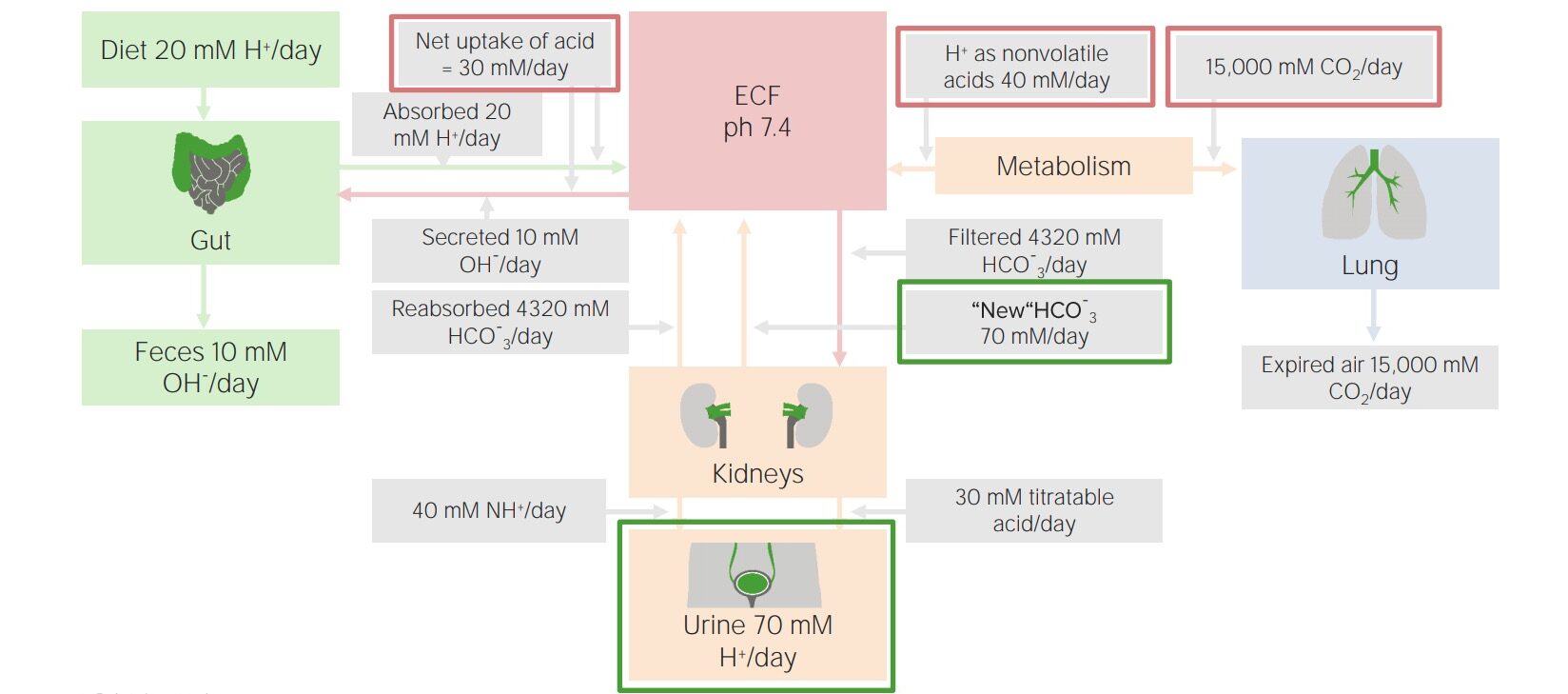
Factors involved in daily acid–base balance
Image by Lecturio.The kidneys Kidneys The kidneys are a pair of bean-shaped organs located retroperitoneally against the posterior wall of the abdomen on either side of the spine. As part of the urinary tract, the kidneys are responsible for blood filtration and excretion of water-soluble waste in the urine. Kidneys: Anatomy are primarily responsible for elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy of the fixed (nonvolatile) acids, approximately 70 mmol daily. They prevent excretion of bicarbonate Bicarbonate Inorganic salts that contain the -HCO3 radical. They are an important factor in determining the ph of the blood and the concentration of bicarbonate ions is regulated by the kidney. Levels in the blood are an index of the alkali reserve or buffering capacity. Electrolytes, and also pair acid excretion with new bicarbonate Bicarbonate Inorganic salts that contain the -HCO3 radical. They are an important factor in determining the ph of the blood and the concentration of bicarbonate ions is regulated by the kidney. Levels in the blood are an index of the alkali reserve or buffering capacity. Electrolytes generation so that the bicarbonate Bicarbonate Inorganic salts that contain the -HCO3 radical. They are an important factor in determining the ph of the blood and the concentration of bicarbonate ions is regulated by the kidney. Levels in the blood are an index of the alkali reserve or buffering capacity. Electrolytes buffering system is always available at full capacity.
Bicarbonate Bicarbonate Inorganic salts that contain the -HCO3 radical. They are an important factor in determining the ph of the blood and the concentration of bicarbonate ions is regulated by the kidney. Levels in the blood are an index of the alkali reserve or buffering capacity. Electrolytes is freely filtered at the glomerulus, and 100% of it is then reabsorbed (80% in the proximal tubule Proximal tubule The renal tubule portion that extends from the bowman capsule in the kidney cortex into the kidney medulla. The proximal tubule consists of a convoluted proximal segment in the cortex, and a distal straight segment descending into the medulla where it forms the u-shaped loop of henle. Tubular System) through the following process:
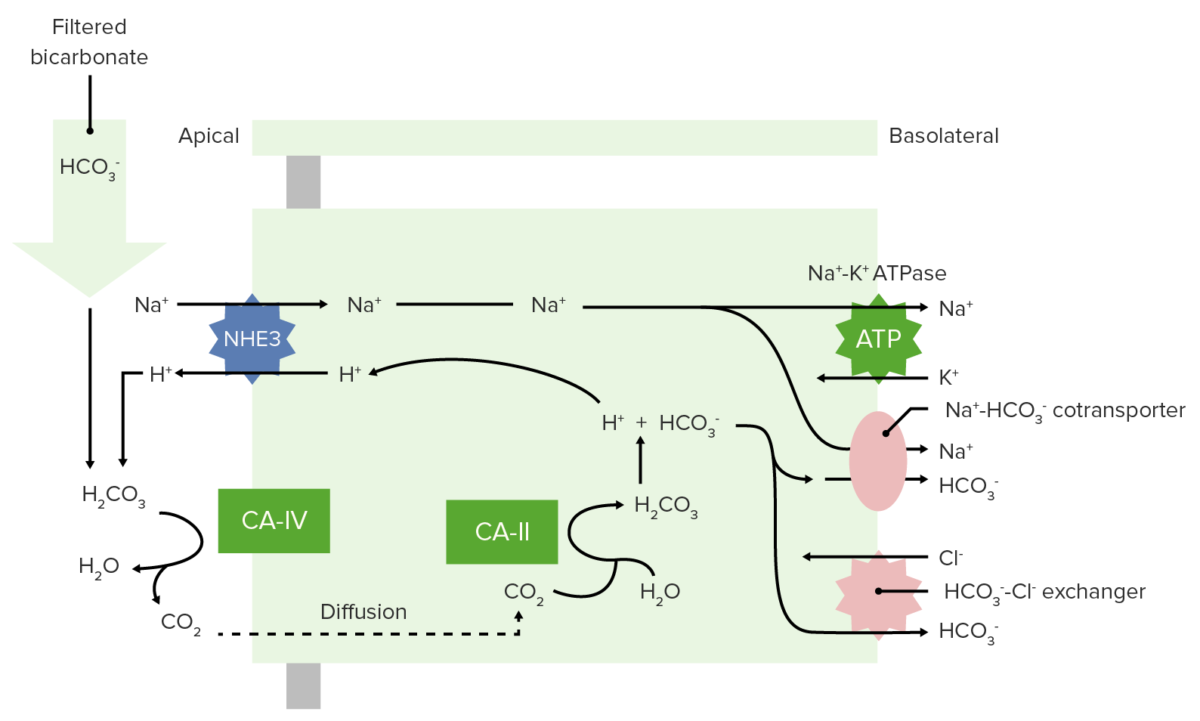
Bicarbonate reabsorption in the proximal tubule
CA-IV: carbonic anhydrase IV
CA-II: carbonic anhydrase II
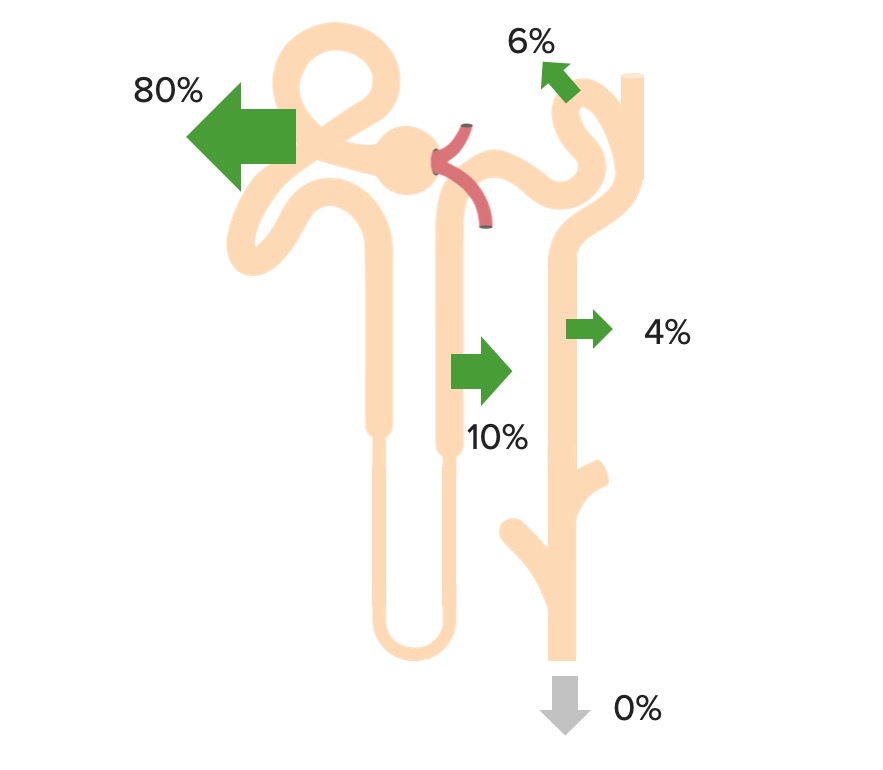
Renal reabsorption of HCO3–
Image by Lecturio.NH3 is able to help excrete fixed acids.
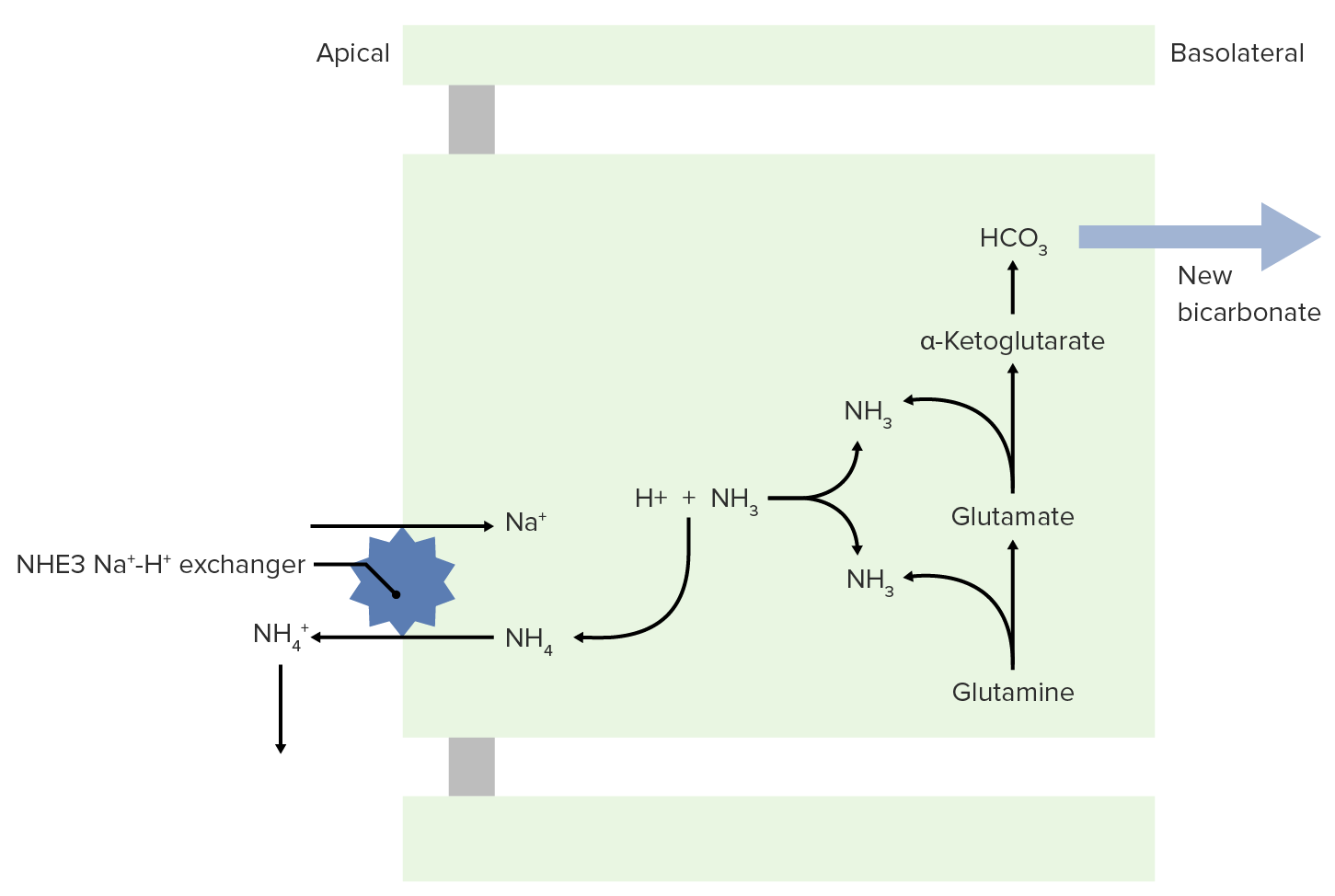
NH3 and NH4+ transport to the lumen for excretion
Image by Lecturio.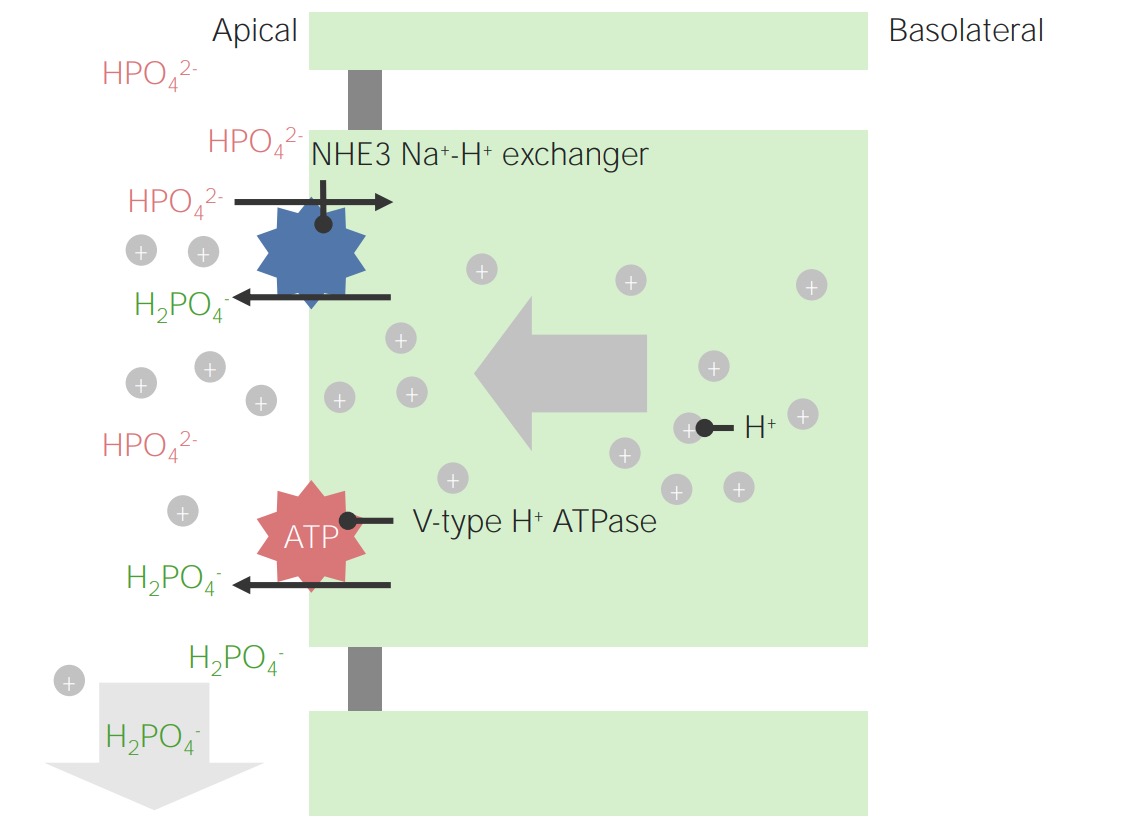
Example of phosphate as a titratable acid:
Note that intracellular H+ ions originate from dissociation of H2CO3. This is also the mechanism for the regeneration of HCO3–.
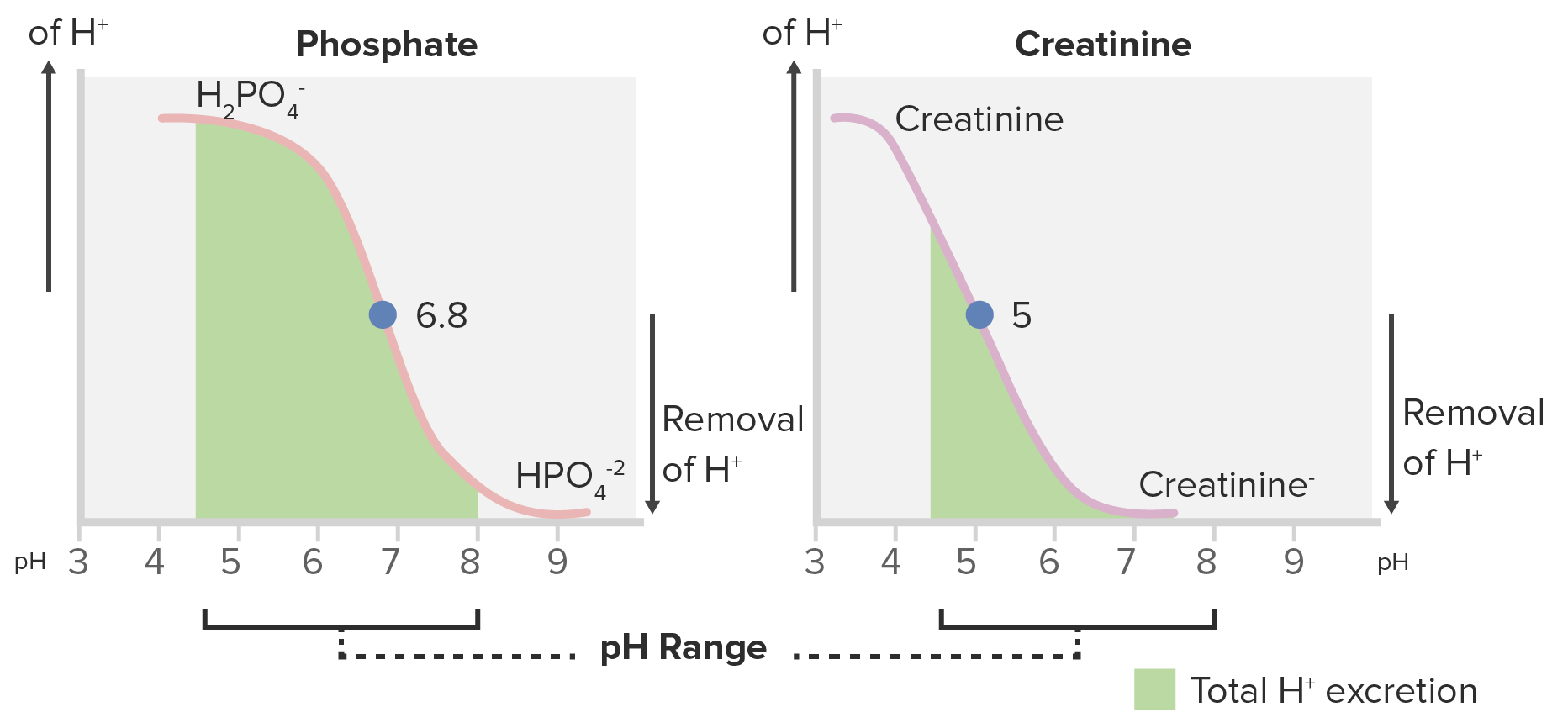
Titration curves for phosphate and creatinine:
Phosphate is a better titratable acid because of its greater area under the curve within the range of the normal urine pH (4.5–8).
When a disease process overwhelms the normal capability to regulate pH, the primary acid–base disorders listed below occur. Compensatory mechanisms also occur, which help to offset the change in pH.