Ionic reactions occur when two compounds react by transferring electrons and, in the process, may form positively or negatively charged ions. This article focused on different ionic reactions, specifically redox and precipitation reactions. For redox reactions, different topics were discussed, namely assigning oxidation state, basics of the redox process and balancing redox reactions. For the precipitation reactions, the article focused more on the solubility rules in predicting the formation of insoluble precipitates in water.
Last updated: Mar 22, 2022
Concentrations of solutions may be expressed in different forms. These include molarity, molality, % weight/volume, % weight/weight, % volume/volume, normality, titer, and much more. The most common concentration quantity for the ionic solution is the molarity. Molarity is expressed as the number of moles Moles Primary Skin Lesions of solute per L of the solution. For example, to compute for the concentration of a sodium Sodium A member of the alkali group of metals. It has the atomic symbol na, atomic number 11, and atomic weight 23. Hyponatremia chloride Chloride Inorganic compounds derived from hydrochloric acid that contain the Cl- ion. Electrolytes solution that is prepared by dissolving 1 g of NaCl in 1.00 L of the solution, the following equation will be used.
Each atom that participates in an oxidation-reduction reaction is assigned an oxidation number that reflects its ability to acquire, donate or share electrons.
Redox is a short-hand notation for reduction-oxidation. These reactions are named Redox reactions because the reaction involves two simultaneous steps which are reduction and oxidation reaction. These are reactions wherein electron transfer is involved between two chemical species. In the process, one of the reactants loses electrons, while the other species gain the electron. The charge is also conserved in the process.
Oxidation state, or number, is the total number of electrons that an atom has gained or has lost to form a chemical bond. Each atom participating in an oxidation-reduction reaction is assigned an oxidation state to determine the ability of each atom to accept, donate or share electrons. Changes in the oxidation states of the atom will reflect whether those atoms were reduced or oxidized in the process. Some general rules for assigning oxidation states are listed below.
In a redox reaction, the compound that loses electrons are termed oxidized and is called the reducing agent. On the other hand Hand The hand constitutes the distal part of the upper limb and provides the fine, precise movements needed in activities of daily living. It consists of 5 metacarpal bones and 14 phalanges, as well as numerous muscles innervated by the median and ulnar nerves. Hand: Anatomy, the compound that accepts electrons during the process is termed reduced and is called the oxidizing agent.
For example, in the reaction of Fe2+ ions with Ce4+ ions:
Fe2+ + Ce4+ → Fe3+ + Ce3+
We can write the reaction above into two half-reaction or steps:
Fe2+ → Fe3+ + 1e– Oxidation
Ce4+ + 1 e– → Ce3+ Reduction
Fe2+ ions lose electrons to produce Fe3+. There is an increase in the oxidation state, and so the process is termed as oxidation. The electrons released by Fe2+ will then be accepted by the Ce4+ producing Ce3+. In the next half-reaction, the oxidation state of Ce becomes lower in the process of reduction. Fe2+ is oxidized in the process while Ce4+ is reduced. In this reaction, Fe2+ is the reducing agent, while Ce4+ is the oxidizing agent.
Like any reactions, redox reactions follow the law of conservation of matter but aside from balancing the elements, overall charges in the reaction are also to be balanced. Balancing redox reactions may be achieved using the steps described below. As an example, consider the reaction of the permanganate ion, MnO4–, and sulfurous acid, H2SO3.
1. The reaction will be divided into 2 half-reactions. To identify the half-reactions, you just need to look at the atoms which changed oxidation states in the reaction.
2. The next step is to balance the atoms beside H and O. Most of the time, these atoms are the ones oxidized or reduced. Since, in the example, there are no differences in the O and H atoms, the half-reactions are retained.
3. The O atoms are then balanced by adding water in either the reactant or the product Product A molecule created by the enzymatic reaction. Basics of Enzymes side.
Al AL Amyloidosis(OH)3 + 3HCI → 3H3O+ + AlCl3 (Base)
Al AL Amyloidosis(OH)3 + OH → Al AL Amyloidosis(OH)4- (Acid)
4. After balancing the O atoms, in most cases, there will be an excess of H-atom on one side. The H atoms are then balanced by adding H+ to either side of the reaction.
5. By this time, it will be noticed that the charges of the half-reactions are not balanced. The next step then is to balance the charges by adding electrons to either side of the reactions.
6. The next step is to add the two half-reactions to get the over-all balanced redox reaction. Since an electron product Product A molecule created by the enzymatic reaction. Basics of Enzymes cannot be yielded in a reaction, each half-reaction will be multiplied by a certain factor so that the electrons will be balanced.
HNO(3aq) + H2O(l) → H3O+(aq) + NO3-(aq)
7. Chemical entities present in the reactant and the product Product A molecule created by the enzymatic reaction. Basics of Enzymes sides of the reaction are canceled. No electron should be present in the final balanced reaction. If the reaction is in acidic medium, you may stop at step no. 7.
8. In a basic medium, the H+ ions are completely neutralized by adding OH- on both sides of the reaction. The OH- combined with the H+ will produce water.
Some ionic reactions lead to the formation of a precipitate or a solid. The stability of the product Product A molecule created by the enzymatic reaction. Basics of Enzymes enables the formation of insoluble precipitates. Precipitates are commonly formed from the interaction of a cation and an anion in solution. When the two ions react with each other, a precipitate may form or not. Below is a list of the common solubility rules.
Sometimes, there is a need to write the Net Ionic Equation (NIE) of a reaction. NIE involves showing the predominant form of a substance when it is in contact with water. For example, a completely soluble salt like NaCl will not exist as solid NaCl in a reaction with water as a solvent. Instead, it will be represented as its ions Na+, and Cl–. Using the NIE, we can emphasize how the precipitates are formed in a reaction.
For example, let us consider the reaction of an aqueous solution of KI KI An inorganic compound that is used as a source of iodine in thyrotoxic crisis and in the preparation of thyrotoxic patients for thyroidectomy. . Antithyroid Drugs and AgNO3. Based on the solubility rules, both compounds are soluble in water. This means both compounds dissociate in water to produce their ionic components. As soon as a drop of KI KI An inorganic compound that is used as a source of iodine in thyrotoxic crisis and in the preparation of thyrotoxic patients for thyroidectomy. . Antithyroid Drugs is added to the AgNO3 solution, a yellow precipitate was formed.
Formula unit equation:
Following the solubility rules, it will only be the AgCl product Product A molecule created by the enzymatic reaction. Basics of Enzymes that will stay as a solid as it is insoluble in water. Using the total ionic equation for the reaction, we can emphasize that it is only the AgCl that is insoluble in our reaction system.
Total ionic equation:
[K+(aq) + I–(aq)] + [ Ag AG Metabolic Acidosis+(aq) + NO3–(aq)] – [K+(aq)+NO3–(aq)] + Agl(s)
As you can see, there are ions that are present in both the reactant and product Product A molecule created by the enzymatic reaction. Basics of Enzymes sides. These ions are called spectator ions and are canceled to obtain the net ionic equation.
Net ionic equation:
Ag AG Metabolic Acidosis+(aq) + I–(aq) → Agl(s)
Imagine a 1.00 L sample of polluted water was analyzed for lead(II) ion, Pb2+ , by adding an excess of sodium Sodium A member of the alkali group of metals. It has the atomic symbol na, atomic number 11, and atomic weight 23. Hyponatremia sulfate to it. The mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast of lead(II) sulfate that precipitated was 229.8 mg.
What is the mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast of lead in a liter of water? Express the answer as mg of lead per liter of solution.
Na2SO4(aq) + Pb2+(aq) → 2Na+(aq) + PbSO4(s)
First, we must obtain the mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast percentage of lead in lead(II) sulfate, by dividing the molar mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast of lead by the molar mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast of PbSO4, then multiplying by 100.
The, calculate the amount of lead in the PbSO4 precipitated.
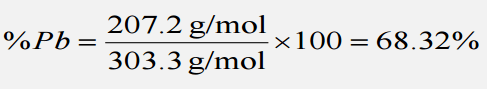
Amount Pb in sample = 229.8 mg PbSO4 x 0.6832 = 157.0 mg Pb