Chemistry is the study of matter and its interactions. Atoms are the basic building blocks of matter, composed of a positively charged nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles containing protons and neutrons, surrounded by negatively charged electrons. The number of protons in an atom's nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles determines its atomic number and its placement on the periodic table of elements. The periodic table arranges elements in order of increasing atomic number, grouping them based on their properties and reactivity. Chemical reactions involve the rearrangement of atoms to form new substances. A redox reaction is a reaction with electron transfer in which oxidation and reduction take place with each other as a partial reaction. A displacement reaction is a type of chemical reaction in which the atom of the more reactive element(s) displaces the atom of the less reactive element(s). An acid–base reaction involves the exchange of one or more hydrogen ions between different chemical species.
Last updated: Mar 31, 2023
Atoms are the basic units of all matter. They consist of a positively charged nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles and a negatively charged shell in which the electrons move in fixed, elliptical orbits.
Atoms are made of three fundamental particles:
| Charge | Mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast/amu | ||
| Nucleons | Protons | +1 | 1.0073 |
| Neutrons | 0 | 1.0087 | |
| Electrons | -1 | 5.4859 x 10-4 |
Atoms and elements are defined by three numbers relating to the composition of the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles:
To properly identify an atom, the chemical name is written as follows:
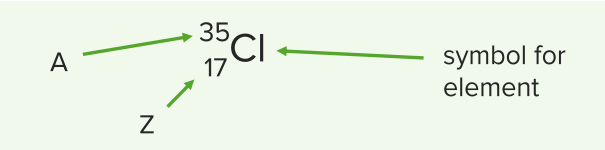
Chemical name of atoms
Image by Lecturio.The atomic core ( nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles) possesses positively charged protons and neutrons, which have no charge. What’s special about the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles? Compared to the electron shell, it is much smaller, but yet it accounts for 99% of the mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast of the whole atom.
The nuclide is a particular type of nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles, which is precisely defined by the number of specific nucleons inside it. Nucleons are a collective term used to refer to subatomic particles inside the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles, which are the protons and the neutrons. Nuclides that have the same number of protons, but a different number of neutrons, are referred to as isotopes of that element.
Isotopes represent atoms of the same element that differ in the number of neutrons. If the mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast number in the periodic table of elements represents a fraction, the naturally occurring isotopes will be taken into account as a percentage. To calculate the mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast number, the following equation is used.
Example: Carbon has an atomic number of 6 (which means each carbon atom has 6 protons and 6 neutrons) and a mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast number of 12.011:
For approximately 20 of the mononuclidic elements (including sodium Sodium A member of the alkali group of metals. It has the atomic symbol na, atomic number 11, and atomic weight 23. Hyponatremia, phosphorus, and fluorine), only one stable isotope exists; these are called monoisotopic elements.
Mixed elements are elements that contain different stable isotopes, such as hydrogen, which contains the stable isotopes protium and tritium.
In the shells of the atom, the electrons move in elliptical orbits around the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles. The electrons fill the shells from the inside to the outside. These shells are named in alphabetical order, starting with the letter K. This means that the first or the innermost shell is called “K,” the second one “L,” and the third one “M,” etc ETC The electron transport chain (ETC) sends electrons through a series of proteins, which generate an electrochemical proton gradient that produces energy in the form of adenosine triphosphate (ATP). Electron Transport Chain (ETC).
Atoms are neutral-charged particles, which means that they possess equal numbers of electrons and protons. If an atom releases an electron as part of a reaction and incorporates other electrons, then it is called a charged atom, which is known as an ion. Positive ions, which are ions with fewer electrons than protons, are referred to as cations Cations Positively charged atoms, radicals or groups of atoms which travel to the cathode or negative pole during electrolysis. Electrolytes. Negative ions, which are ions with an excess of electrons, are known as anions Anions Negatively charged atoms, radicals or groups of atoms which travel to the anode or positive pole during electrolysis. Electrolytes.
Ions of the main elements follow the octet rule to attain noble gas configuration.
The periodic table is arranged based on the atomic structure of the elements, reflecting their structure and properties while also displaying other values, such as electronegativity.
The periodic table is read from top to bottom and from left to right, following a sequence of increasing atomic numbers. The atomic number represents the number of protons an atom of a particular element possesses.
The periodic table is called as such because the elements are horizontally divided into rows called “periods,” which indicate the total number of electron shells. For example, carbon is in the second period, so it has two electron shells. The table is also divided vertically into columns called “groups,” which indicate the number of electrons present in the outermost shell. To follow our example, carbon is in the fourth group; this means carbon and all the other elements in that group have four electrons in their outermost shell.
Periodic system of the elements
Image: “Periodic table : chemical symbols, atomic numbers, standard atomic weights and names in German” by Cdang. License: CC BY-SA 3.0The simplest orbital is the s orbital (I=0), which is a sphere.
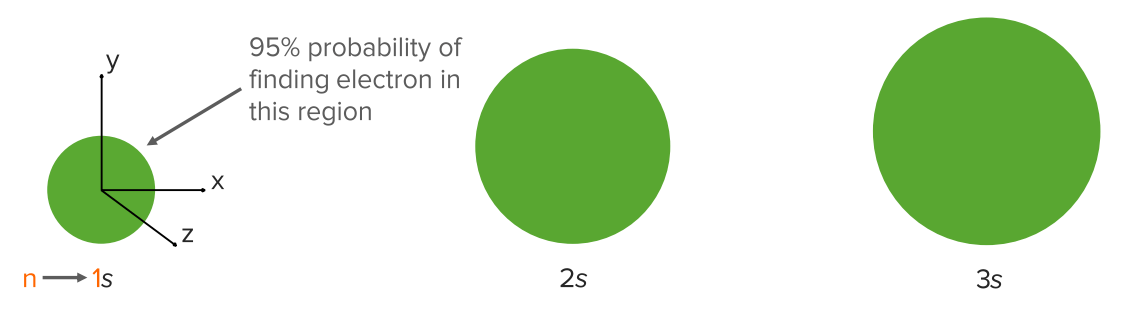
s Orbitals:
1s is an s orbital in shell 1.
2s is an s orbital in shell 2.
Shell 1 (n = 1) has only the 1s orbital.
p Orbitals (I=1) are more complex than s orbitals and have three different types:
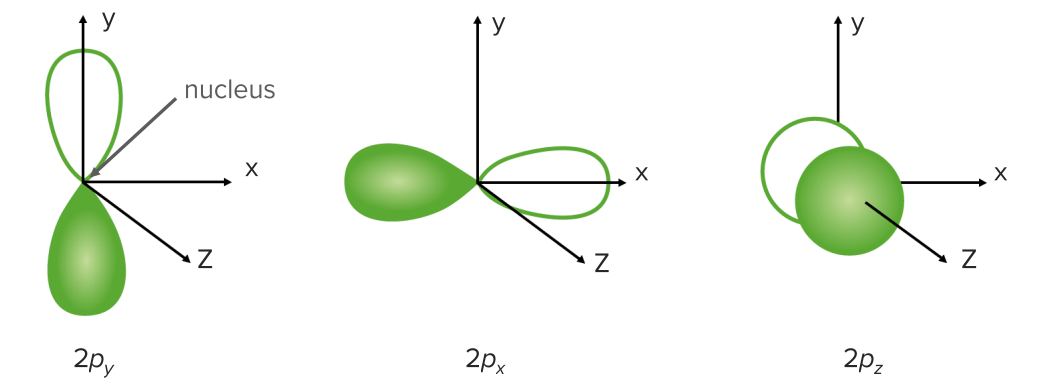
p Orbitals:
Shell 2 (n = 2) has the 2s orbital, which contains 2 electrons, and three 2p orbitals, which contain 6 electrons.
A p orbital has two regions where the electrons may be found on either side of the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles:
This originates from the wave function that defines the orbital.
Note: The main group and the period uniquely determine the location of each element in the periodic table.
Metals can be found on the left at the bottom of the periodic table; nonmetals are on the right at the top.
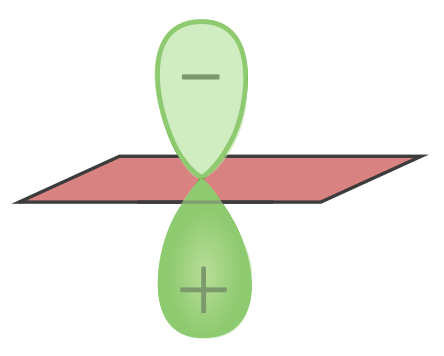
p Orbital with nodal plane and lobe
Image by Lecturio.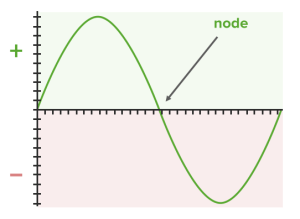
Wave function defining the orbital:
Think of a wave with positive and negative regions. In contrast, the d and f orbitals play an important role in the subgroup elements because their partial occupation decisively influences the properties of transition elements (see electron configuration).
In 1869, Mendeleev developed the law of periodicity based on the regular Regular Insulin repetition of similar characteristics of elements. The periodicity described by him is based on the internal structure of atoms and their electron shells in particular.
The periods and the main groups reflect the following rules:
This means that, in a connection between different atoms, the electronegative partner pulls the electron pair in its direction so that the electron pair is no longer located symmetrically between the participating atoms. The electronegativity decreases in these groups because the effective nuclear charge decreases by shielding of the inner orbitals.
The subgroup elements represent the transition metals. The essential biochemical subgroup elements (e.g., zinc Zinc A metallic element of atomic number 30 and atomic weight 65. 38. It is a necessary trace element in the diet, forming an essential part of many enzymes, and playing an important role in protein synthesis and in cell division. Zinc deficiency is associated with anemia, short stature, hypogonadism, impaired wound healing, and geophagia. It is known by the symbol zn. Trace Elements, iron Iron A metallic element with atomic symbol fe, atomic number 26, and atomic weight 55. 85. It is an essential constituent of hemoglobins; cytochromes; and iron-binding proteins. It plays a role in cellular redox reactions and in the transport of oxygen. Trace Elements, manganese Manganese A trace element with atomic symbol mn, atomic number 25, and atomic weight 54. 94. It is concentrated in cell mitochondria, mostly in the pituitary gland, liver, pancreas, kidney, and bone, influences the synthesis of mucopolysaccharides, stimulates hepatic synthesis of cholesterol and fatty acids, and is a cofactor in many enzymes, including arginase and alkaline phosphatase in the liver. Trace Elements) are called trace elements Trace elements Trace elements are minerals required in small amounts (1-100 mg/day in adults) to carry out biologic functions. These elements act as cofactors for essential enzymes as well as being components of hormones and antioxidant molecules. Iron, chromium, copper, and iodine are among these elements. Trace Elements and should be periodically taken in small amounts by humans. These are important for the function of metalloenzymes.
Atoms can be illustrated in diagrams featuring other characteristics, such as the energy level scheme and the electron configuration.
The electron configuration displays the distribution of electrons in the atomic orbitals. It is specific for each atom.
Note: An orbital is a region within the atom where the electrons are highly likely to be found. Each orbital contains a maximum of 2 electrons.
The electron configuration is established by listing each orbital. The following factors must be considered:
Is it a main element or a subgroup element?
→ For main group elements, the last occupied orbital is an s orbital or a p orbital.
→ For subgroup elements, the last occupied orbital is a d orbital or an f orbital.
In which period is the atom?
→ The highest level corresponds to the period in which the atom is located.
Atomic number
→ The number of electrons in a neutral atom equals the atomic number.
Electron configurations follow certain rules:
Energy levels/orbitals are stable when they are full, empty, or half full.
Looking at the electron configuration also will tell you the oxidation number of the atom.
The oxidation number represents the amount of electrons that have been accepted or released by an atom compared to its neutral elemental state.
The oxidation number indicates the ionic charge (valence).
The goal of each atom is to reach a noble gas configuration. To achieve this, the atoms will either release or accept electrons.
Note: The noble gas configuration is an energetically stable arrangement of electrons in which the outer shell is fully occupied with electrons (octet rule). The atoms of all elements try to achieve this state. It has been achieved by the elements of the 8th main group (noble gases), which is why they are inert.
To determine the oxidation number of an atom in a substance, a number of principles and rules must be observed:
Evidence: If several of the atoms given in this list occur at once, the oxidation numbers are listed according to this hierarchy.
Example: H2O2 → H: +1; O: -1 (the oxidation number of hydrogens stands in the hierarchy above the oxidation number of oxygen; therefore, hydrogen receives its oxidation number as given in the list above)
General determinations
A substance can be solid, liquid, or gas.
This means the change of a state of matter.
During a change in the state of matter, two phases simultaneously coexist (e.g., ice-water mixture, water-steam).
Energy is always consumed during a change of state. If energy is supplied during a phase change in one direction, energy will be released during a change in the other direction.
The following common terms should be known:
The pH pH The quantitative measurement of the acidity or basicity of a solution. Acid-Base Balance scale Scale Dermatologic Examination is a measure of hydrogen ion (H+) concentration, and it is a logarithmic scale Scale Dermatologic Examination.
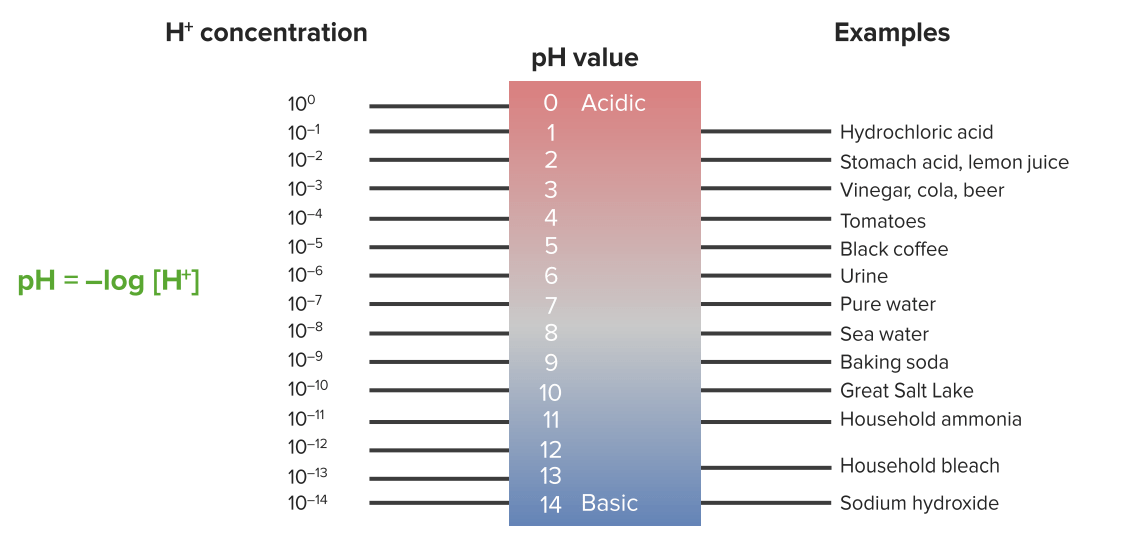
The pH scale
Image by Lecturio.Water dissociates into H+ and OH– in equal amounts and thus is neutral.
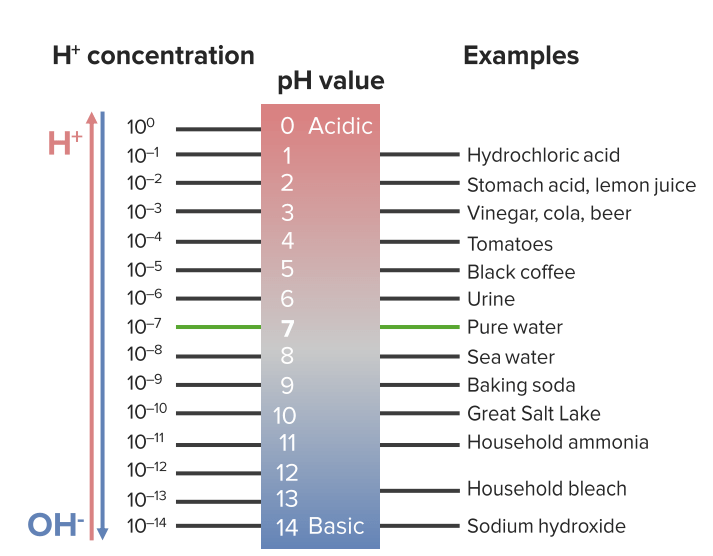
The pH scale: acidity and basicity
Image by Lecturio.Buffers Buffers A chemical system that functions to control the levels of specific ions in solution. When the level of hydrogen ion in solution is controlled the system is called a ph buffer. Acid-Base Balance help cells maintain pH pH The quantitative measurement of the acidity or basicity of a solution. Acid-Base Balance homeostasis Homeostasis The processes whereby the internal environment of an organism tends to remain balanced and stable. Cell Injury and Death.
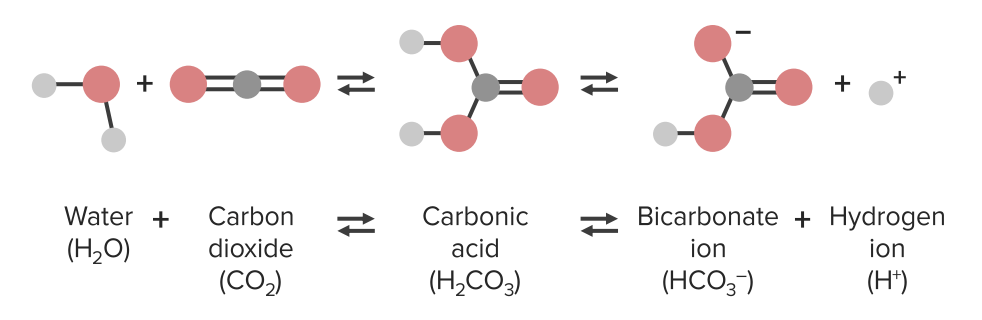
pH homeostasis
Image by Lecturio.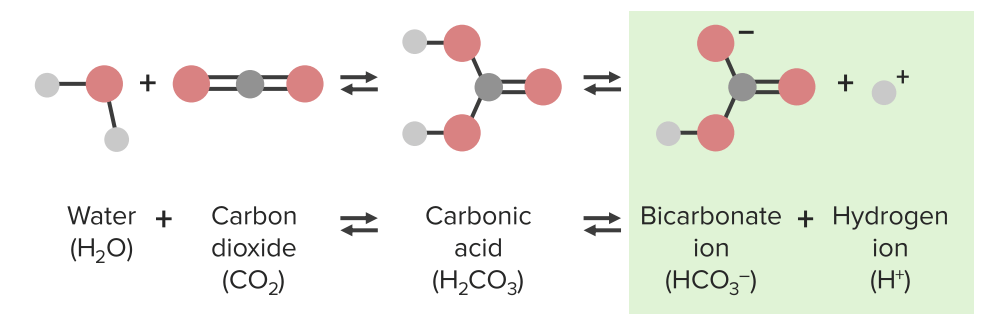
pH homeostasis
Image by Lecturio.Chemical substances react while forming or dissolving bonds. If forward and reverse reactions plateau Plateau Cardiac Physiology after a certain time, there will be a balance of products and reagents.
Important reaction types in inorganic systems are the redox reaction and the acid-base reaction.
Note: Bronsted acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance are compounds that can donate a proton.
On the other hand Hand The hand constitutes the distal part of the upper limb and provides the fine, precise movements needed in activities of daily living. It consists of 5 metacarpal bones and 14 phalanges, as well as numerous muscles innervated by the median and ulnar nerves. Hand: Anatomy, a Bronsted base can accept protons, which means it acts as a proton acceptor.
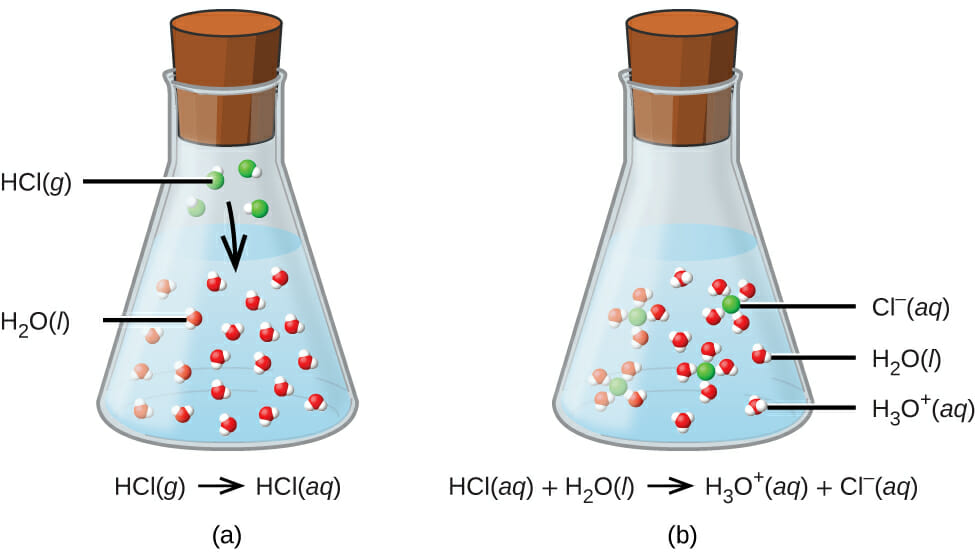
Acid-base reactions
Image: “When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and solvated chloride ions)” by OpenStax College. License: CC BY 4.0Example:
A special form of the acid-base reaction is neutralization. Neutralization is a chemical reaction in which hydrogen ions and hydroxide ions combine with each other to form water.
Word equation: hydroxide + acid → salt + water
Note: It is often assumed that an equimolar amount of acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance and bases Bases Usually a hydroxide of lithium, sodium, potassium, rubidium or cesium, but also the carbonates of these metals, ammonia, and the amines. Acid-Base Balance automatically react with each other and form a neutral solution. However, not all of these types of reactions produce a neutral solution. This is only true for strong acid and strong base reactions. If you react a strong acid with a weak partner, an acidic solution will be produced. If you react a strong base with a weak partner, a basic solution will be produced.
The redox reaction is a reaction with electron transfer in which oxidation and reduction take place with each other as a partial reaction. During oxidation, atoms donate electrons, leading to a more positive oxidation state. During reduction, atoms accept electrons, leading to a more negative oxidation state.
The precondition for a redox reaction is the presence of two corresponding electron pairs.
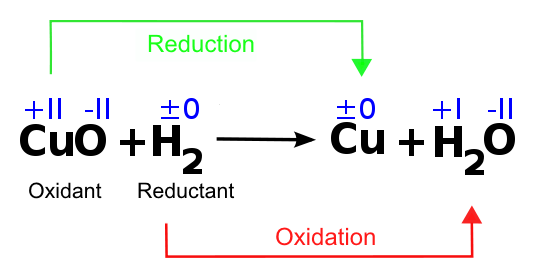
Example of a redox reaction
Image by Lecturio.As shown in the example, CuO is reduced and afterward called an oxidant. Furthermore, it is shown that the oxidation number of copper Copper A heavy metal trace element with the atomic symbol cu, atomic number 29, and atomic weight 63. 55. Trace Elements changes, while the oxidation number of oxygen stays the same. This means that the atom of copper Copper A heavy metal trace element with the atomic symbol cu, atomic number 29, and atomic weight 63. 55. Trace Elements is the part of the copper Copper A heavy metal trace element with the atomic symbol cu, atomic number 29, and atomic weight 63. 55. Trace Elements oxide-compound that accepts electrons and therefore is involved in the electron transfer.
H2 is oxidized, which means that the electrons will be released, and it acts as a reductant.
Summary: The acid-base-reaction, as well as the redox reaction, are donor-acceptor reactions. The difference is that, in acid-base-reactions, protons are transferred; in redox reactions, electrons are transferred.
The basic requirement for all chemical calculations is knowledge of essential measurements and fundamental units, which are known as SI units (Système International d’unités).
Unit of atomic mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast, u: atomic mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast is often expressed in units of daltons (Da)
Amount of substance, n: 1 mol = 6 × 1023 → n = m/M
Mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast, m: 1 kg
Molar mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast, M: 1 g/mol → M = m/n
Substance concentration, c: 1 mol/cm3
Volume, V: 1 m³
The LMA provides the mathematical basis for calculating the ratio between products and reactants in an adjusted balance based on the prevailing substance concentration. The ratio K is constant for a specific reaction with the same conditions.
Assumptions:
K = equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy/ mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast action constant
Stoichiometric numbers of reaction numbers must be considered as an exponent in the LMA.
Calorimetry deals with measurements of the released or absorbed heat Heat Inflammation quantities of chemical reactions.
Assumptions:
Equation for calorimetric calculations:
[cp H2O = 4.19 kJ × kg-1 × K-1]
[RP = reaction product]
This theorem states that the reaction enthalpy Enthalpy Enzyme Kinetics depends only on the initial and final condition and is independent of the reaction pathway.
If it is not possible to measure the reaction enthalpy Enthalpy Enzyme Kinetics directly, it is feasible to determine it indirectly by using the Hess theorem. For this method, the enthalpies of the chemical reactions can be calculated by the listed enthalpy Enthalpy Enzyme Kinetics of formation and enthalpy Enthalpy Enzyme Kinetics of combustion.
Note: The molar reaction enthalpy Enthalpy Enzyme Kinetics is the converted energy of a chemical reaction, based on the amount of substance (unit: kJ/mol).
pH-value calculation
The pH pH The quantitative measurement of the acidity or basicity of a solution. Acid-Base Balance value is the negative decadic logarithm of the hydronium concentration (at 25°C) and therefore is a measure of how acidic or basic a solution is.
This calculation is particularly relevant for acid-base reactions.
Example:
Given: 0.2 mol/L HCl HCL Hairy cell leukemia (HCL) is a rare, chronic, B-cell leukemia characterized by the accumulation of small mature B lymphocytes that have “hair-like projections” visible on microscopy. The abnormal cells accumulate in the peripheral blood, bone marrow (causing fibrosis), and red pulp of the spleen, leading to cytopenias. Hairy Cell Leukemia solution.
Since HCl HCL Hairy cell leukemia (HCL) is a rare, chronic, B-cell leukemia characterized by the accumulation of small mature B lymphocytes that have “hair-like projections” visible on microscopy. The abnormal cells accumulate in the peripheral blood, bone marrow (causing fibrosis), and red pulp of the spleen, leading to cytopenias. Hairy Cell Leukemia is a strong acid, the concentration of the acid is equal to the concentration of H+.
pH pH The quantitative measurement of the acidity or basicity of a solution. Acid-Base Balance = −log [H+] = −log [0.2] = 0.7
→ 0.7 < 7 (this means that it is a highly acidic solution).