What are acid–base disorders?
Acid-base disorders involve imbalances in the body’s pH levels, often categorized as acidosis (low pH) or alkalosis (high pH). These disorders can be respiratory or metabolic in origin, and they are commonly identified through arterial blood gas (ABG) analysis.
What does compensation mean?
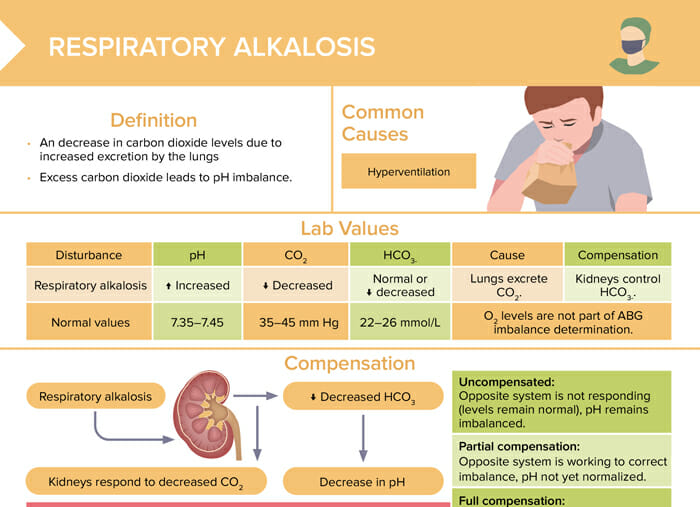
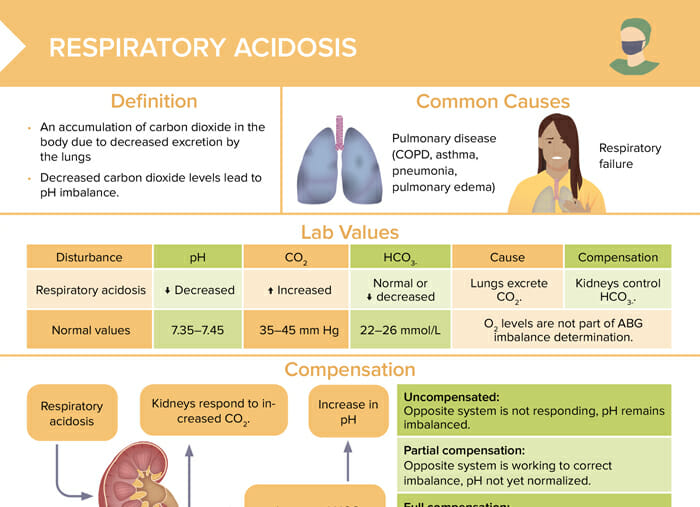
Uncompensated:
Opposite system is not responding, pH remains imbalanced.
Partial compensation:
Opposite system is working to correct the imbalance, pH not yet normalized.
Full compensation:
Homeostasis achieved, all lab values return to normal.
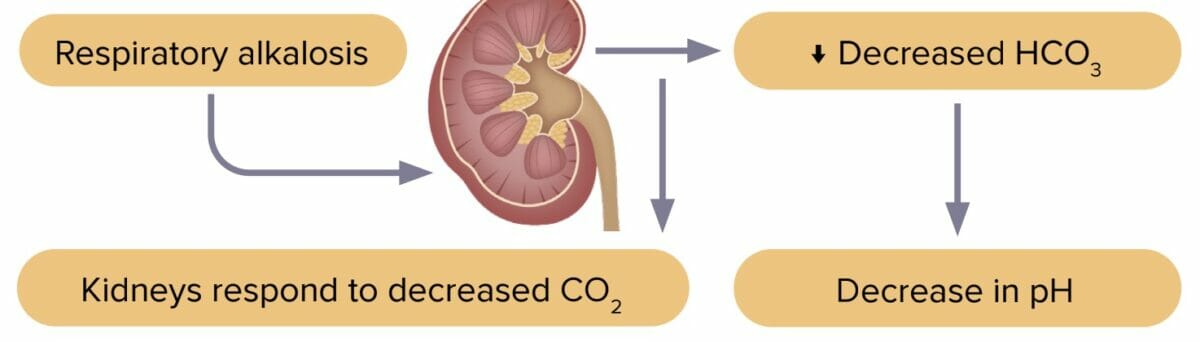
Respiratory alkalosis
Respiratory alkalosis is a decrease in carbon dioxide levels due to increased excretion by the lungs. Excess carbon dioxide leads to pH imbalance.
The most common cause of respiratory alkalosis is hyperventilation.
- Cause: Lungs excrete CO2.
- Compensation: Kidneys control HCO3-.
- O2 levels are not part of ABG imbalance determination.
Lab values for respiratory alkalosis
| Normal values | Respiratory alkalosis | |
| pH | 7.35–7.45 | Increased |
| CO2 | 35–45 mm Hg | Decreased |
| HCO3- | 22–26 mmol/L | Normal or decreased |
Compensation in respiratory alkalosis
Nursing tip: Kussmaul breathing is an abnormal rapid, deep breathing pattern that helps the body blow off extra CO2; often seen in DKA.
Treatment of respiratory alkalosis
- Fix the underlying cause to slow breathing
- Address anxiety and assist client with relaxation
- Renal system decreases HCO3-
Free download: respiratory alkalosis at a glance
Reviews definition, causes and lab values and treatment of respiratory alkalosis
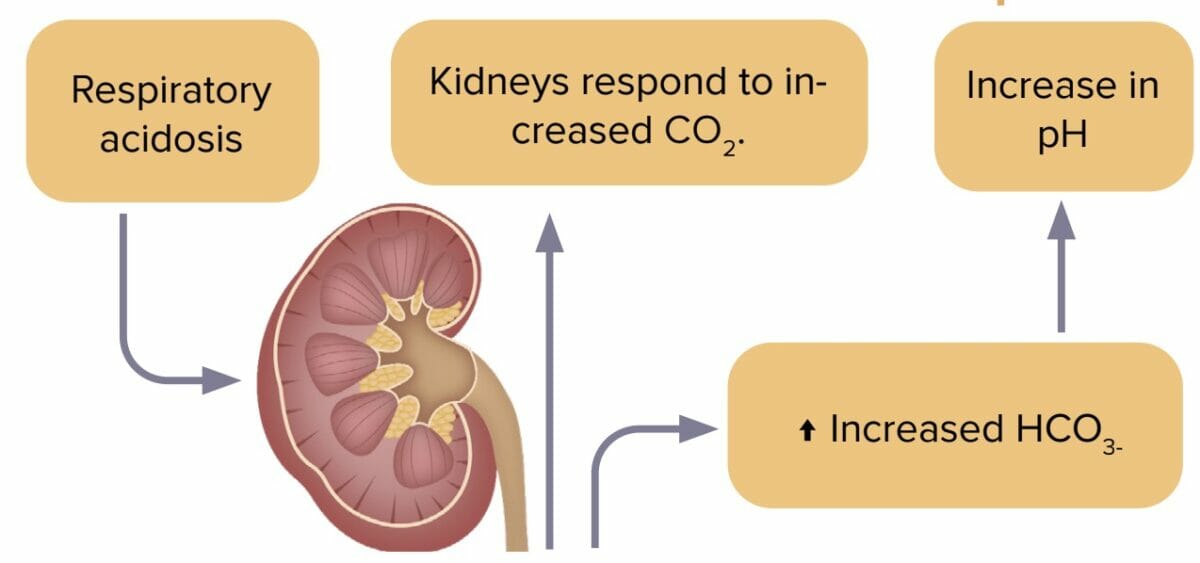
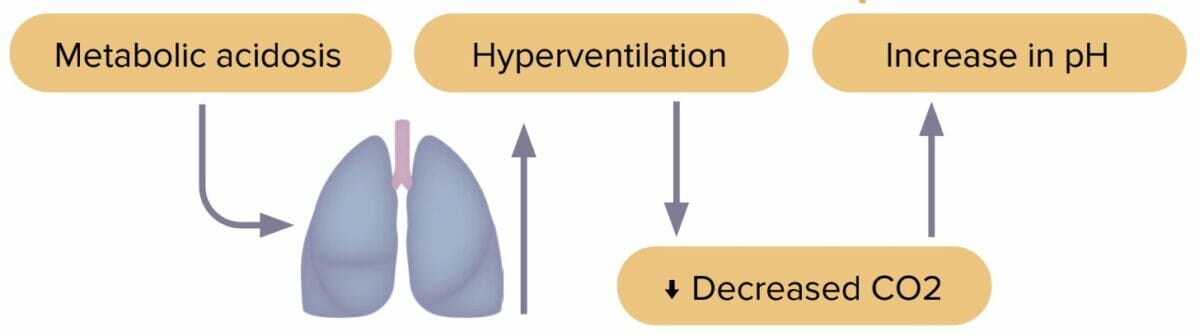
Respiratory acidosis
Respiratory acidosis is an accumulation of carbon dioxide in the body due to decreased excretion by the lungs. Decreased carbon dioxide levels lead to pH imbalance.
The most common causes of respiratory acidosis are:
- Pulmonary disease (COPD, asthma, pneumonia, pulmonary edema)
- Respiratory failure
Lab values for respiratory acidosis
| Normal values | Respiratory acidosis | |
| pH | 7.35–7.45 | Decreased |
| CO2 | 35–45 mm Hg | Increased |
| HCO3- | 22–26 mmol/L | Normal or decreased |
Compensation in respiratory acidosis
Treatment of respiratory acidosis
- Medication: bronchodilators, steroids
- BiPAP or ventilator to assist breathing
- Renal system increases HCO3-.
Free download: respiratory acidosis at a glance
Reviews definition, causes and lab values and treatment of respiratory acidosis
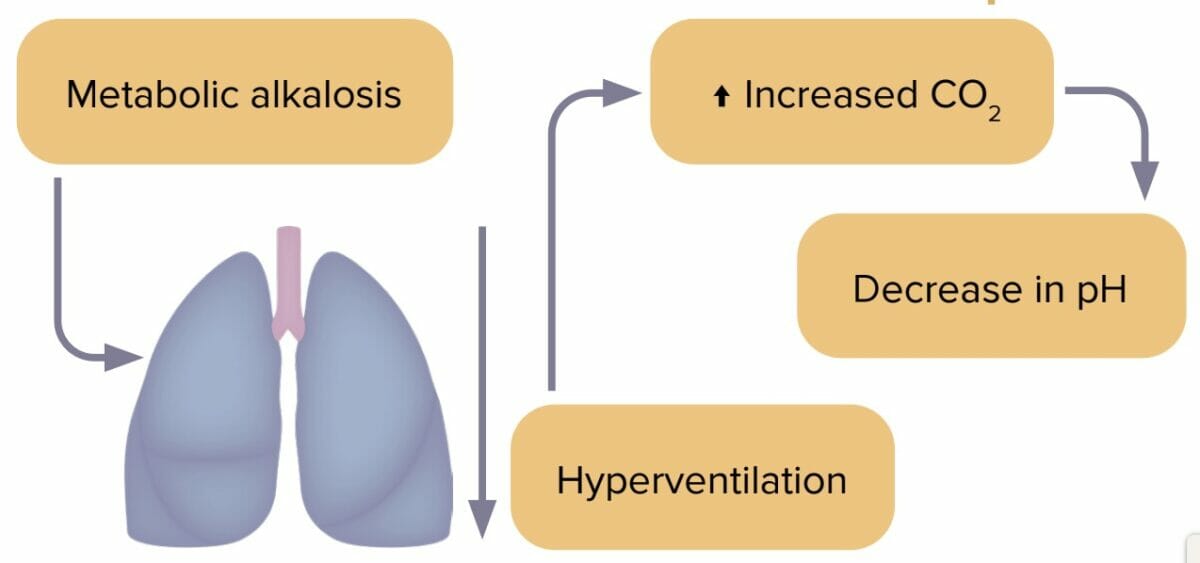
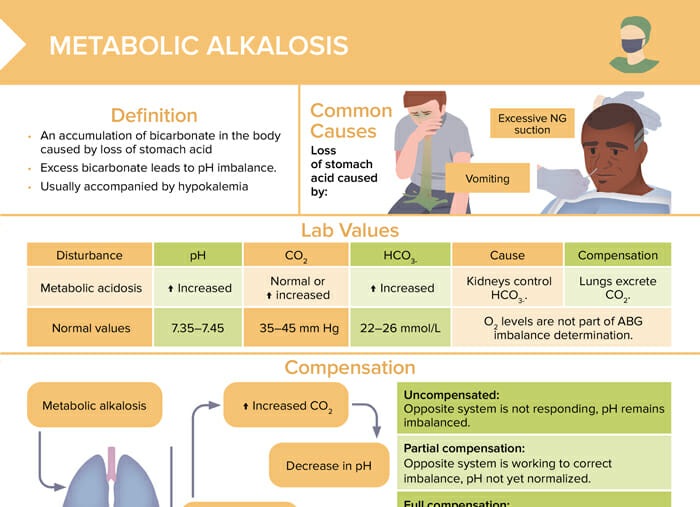
Metabolic alkalosis
Metabolic alkalosis is an accumulation of bicarbonate in the body caused by loss of stomach acid. Excess bicarbonate leads to pH imbalance. Metabolic alkalosis is usually accompanied by hypokalemia.
The most common cause of metabolic alkalosis is a loss of stomach acid, caused by excessive NG suction or vomiting.
Lab values for metabolic alkalosis
| Normal values | Metabolic alkalosis | |
| pH | 7.35–7.45 | Increased |
| CO2 | 35–45 mm Hg | Normal or increased |
| HCO3- | 22–26 mmol/L | Increased |
Compensation in metabolic alkalosis
Treatment of metabolic alkalosis
- Fix the underlying cause
- Determine if IV fluids are needed for volume replacement
- Body decreases respiratory rate to decrease CO2.
Free download: metabolic alkalosis at a glance
Reviews definition, causes and lab values and treatment of metabolic alkalosis
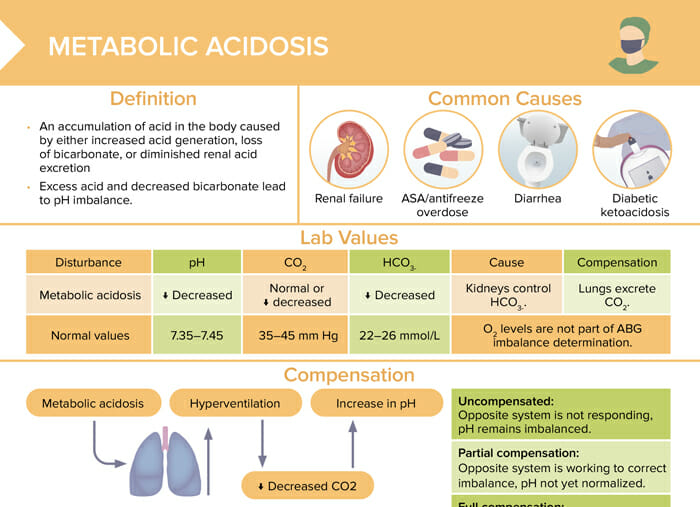
Metabolic acidosis
Metabolic acidosis is an accumulation of acid in the body caused by either increased acid generation, loss of bicarbonate, or diminished renal acid excretion. Excess acid and decreased bicarbonate lead to pH imbalance.
The most common causes of metabolic acidosis are:
- Renal failure
- ASA/antifreeze overdose
- Diarrhea
- Diabetic ketoacidosis
Lab values for metabolic acidosis
| Normal values | Metabolic acidosis | |
| pH | 7.35–7.45 | Decreased |
| CO2 | 35–45 mm Hg | Normal or decreased |
| HCO3- | 22–26 mmol/L | Decreased |
Compensation in metabolic acidosis
Treatment of metabolic acidosis
- Fix the underlying cause
- Consider sodium bicarbonate IV
- Body increases respiratory rate to decrease CO2.
Free download: metabolic acidosis at a glance
Reviews definition, causes and lab values and treatment of metabolic acidosis
Identifying acid–base imbalances: examples
- Identify pH (acidosis or alkalosis).
- Identify CO2 (↑, ↓, normal).
- Identify HCO3 (↑, ↓, normal).
- Which label matches pH?
- Look at opposite system not causing the problem, evaluate if it is bringing pH back to normal.
Example #1
| Disturbance | pH | CO2 | HCO3- |
| ??? | 7.48 | 27 | 19 |
Answer: Metabolic acidosis partially compensated.
Example #2
| Disturbance | pH | CO2 | HCO3- |
| ??? | 7.30 | 55 | 25 |
Answer: Respiratory acidosis uncompensated.
Example #3
| Disturbance | pH | CO2 | HCO3- |
| ??? | 7.47 | 47 | 30 |
Answer: Metabolic acidosis partially compensated.
Example #4
| Disturbance | pH | CO2 | HCO3- |
| ??? | 7.25 | 32 | 18 |
Answer: Metabolic acidosis partially compensated.
Mixed acid–base disorders
Mixed acid-base disorders occur when a patient has more than one primary acid-base imbalance simultaneously. For example, a patient could have both respiratory acidosis and metabolic alkalosis. These disorders are often more complex to diagnose and manage, requiring careful interpretation of arterial blood gas (ABG) results alongside clinical assessment.
Acid–base disorders chart
| Normal | Respiratory acidosis | Respiratory alkalosis | Metabolic acidosis | Metabolic alkalosis | |
| pH | 7.35–7.45 | Decreased | Increased | Decreased | Increased |
| CO2 | 35–45 mm Hg | Increased | Decreased | Normal or decreased | Normal or increased |
| HCO3- | 22–26 mmol/L | Normal or decreased | Normal or decreased | Decreased | Increased |