Matter is made up of atoms, and atoms are made up of particles. The particles include protons, neutrons, and electrons of which the protons and neutrons make up the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles. The electrons exist in a cloud orbiting the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles. The electronic structure explains the location and movement of the electrons in atoms. In addition, these electrons exist and move in many different configurations allowing for the existence of different atoms.
Last updated: Dec 15, 2025
The atom is made up of positive, negative, and neutral particles. The nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles, located in the center of the atom, consists of neutrons and protons.
Several theories have been put forward to explain how particles exist in the atom. One idea, the plum pudding model, suggests the positive charge (proton) is distributed throughout the atom, while the negative charges (electrons) appear randomly.
Further investigation by other scientists, namely Niels Bohr and Ernest Rutherford, disproved the plum pudding model. Their research Research Critical and exhaustive investigation or experimentation, having for its aim the discovery of new facts and their correct interpretation, the revision of accepted conclusions, theories, or laws in the light of newly discovered facts, or the practical application of such new or revised conclusions, theories, or laws. Conflict of Interest showed that the positive charge of the atom existed only in the center of the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles. In addition, negative charges (electrons) orbited the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles. Electrons orbit Orbit The orbit is the cavity of the skull in which the eye and its appendages are situated. The orbit is composed of 7 bones and has a pyramidal shape, with its apex pointed posteromedially. The orbital contents comprise the eye, extraocular muscles, 5 cranial nerves, blood vessels, fat, the lacrimal apparatus, among others. Orbit and Extraocular Muscles: Anatomy stably (without radiating) and only in specific orbits. It is a semi-classical model, in which the electron’s motion around the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles is restricted by the quantum rule. In this model, electrons can gain or lose energy if they jump from an orbit Orbit The orbit is the cavity of the skull in which the eye and its appendages are situated. The orbit is composed of 7 bones and has a pyramidal shape, with its apex pointed posteromedially. The orbital contents comprise the eye, extraocular muscles, 5 cranial nerves, blood vessels, fat, the lacrimal apparatus, among others. Orbit and Extraocular Muscles: Anatomy to another.
The electrons exist in the atom in orbitals and in discrete, distinct energy levels. The atom has different energy levels for the electrons to occupy. The energy level is denoted by the letter n, which can only be an integer starting at 1 and incremented by 1. So, n can equal 1, 2, 3, etc ETC The electron transport chain (ETC) sends electrons through a series of proteins, which generate an electrochemical proton gradient that produces energy in the form of adenosine triphosphate (ATP). Electron Transport Chain (ETC).
In addition, there can only be a certain number of electrons at each energy level. The maximum number of electrons at each level is denoted by 2n2. Thus, at the first energy level, when n=1, there can be 2 (1)2 = 2 electrons. At the second energy level, when n=2, there can be 2 (2)2 = 8 electrons. The lowest energy level, i.e., when n=1, is referred to as the ground state.
Electrons can move from one energy level to another. For example, the hydrogen atom has one proton, one neutron, and one electron, which is located in the ground state n=1. This electron can go to a higher state, implying it leaves the n=1 orbital and enters n=2 orbital. Any source of energy, including electricity, allows electrons to move to lower or higher states.
When the electron accomplishes that task, it is then considered to be in an excited state, when it contains more energy. The electron can only achieve this migration by gaining energy to move to a higher state. This can take place when the electron receives a photon of light. It is important to note that electrons usually occupy the lowest available space when in a grounded state.
An electron can also drop to a lower energy state, thus releasing a photon. The photon energy will be equal to the energy the atom loses during migration.
The first equation demonstrates that photon energy is equal to the energy in the excited state minus the energy in the grounded state. The second equation shows another way to calculate the energy of the photon-based on h = Planck’s constant, f = frequency, ʎ = wavelength and c = speed of light.
ΔEatom = Eexcited – Eground = Ephoton
Ephoton = hƒ = hc / λ
The energy needed or released as electrons become excited or head toward the grounded state, respectively, is not equal between the levels. From the ground state up, the space from one energy level to the next decreases. So if an electron goes from level 4 to level 3, it will release Release Release of a virus from the host cell following virus assembly and maturation. Egress can occur by host cell lysis, exocytosis, or budding through the plasma membrane. Virology less energy than it would if it had gone from level 3 to level 2.
The electron can go to higher and higher excited states. Each state has higher and higher energy. This can be calculated with the following equation:
En = (–13.6/n2)eV
In this equation, e = electron charge, V = voltage, n = level and En = energy. Substituting this equation will help calculate the change in the electron’s energy as it traverses from one level to another. This will help determine the emitted photon’s energy.
The calculated energy is negative when referring to bound electrons, which are closer to the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles and bound to the proton. As an electron goes to higher levels, the energy becomes less negative by increasing in value. The higher the level, the more the energy heads towards 0 since the electron is far from the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles, which would occur when n = ∞. Electrons have a constant feeling of energy as long as they are orbiting within the same level. The energy changes when they move higher or lower.
The electron that travels to a lower energy level will emit a photon with a specific frequency and wavelength. Depending on the drop in the energy level, the emitted photon may have a different color. If the energy drop is small, the small energy release Release Release of a virus from the host cell following virus assembly and maturation. Egress can occur by host cell lysis, exocytosis, or budding through the plasma membrane. Virology will cause a low-frequency photon to give off a red color. If the energy drop is large, the large energy release Release Release of a virus from the host cell following virus assembly and maturation. Egress can occur by host cell lysis, exocytosis, or budding through the plasma membrane. Virology will cause a high-frequency photon to give off a blue/purple color.
This process creates a fingerprint for atoms. The emitted energy and light can be directed towards a prism, which separates the light into bands of different colors creating a unique emission spectrum for each atom.
In the opposite manner, light can travel through an atom, where electrons get excited, and the exiting light can be refracted in the prism to see what colors are missing since those frequencies were absorbed by the electrons. This would create a unique absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption spectrum for each atom.
Quantum numbers are used to describe the orbitals in which electrons can be found. The first one is n, which signifies the different energy levels the electrons can occupy. On solving the Schrodinger equation, one can attain the wave function that allows them to calculate the probability Probability Probability is a mathematical tool used to study randomness and provide predictions about the likelihood of something happening. There are several basic rules of probability that can be used to help determine the probability of multiple events happening together, separately, or sequentially. Basics of Probability of which energy level electrons are in an atom.
Each of these levels has different possible shapes. The shapes, or orbitals, are denoted by the letter l. The higher the level, the greater the number of shapes, but this number is limited per level by n-1. For example, when n = 2, l = 0 and 1. At n = 2, there are two possible shapes: circular or bilobed. Each shape can have a different possible orientation Orientation Awareness of oneself in relation to time, place and person. Psychiatric Assessment, denoted by the letter ml. The orientations are also limited and range from –l to +l. The last quantum number is spin ms which can be only +1 or -1, respectively.
The Pauli exclusion principle states that no two electrons can have the same four quantum numbers. As long as one of the quantum numbers is different, the principle is being followed. For example, there are two electrons in an atom with the following numbers: 2, 0, 0 +1, and 2, 0, 0, -1. Both of these possibilities can exist since the spin number is different. A maximum of two electrons can occupy an orbital, and their spins must be different.
The electrons travel in different orbitals based on the quantum number.
The shapes of the different orbitals allow for creating a unique notation for each atom. If an atom is in the first energy level, n = 1, then l = 0 implies an s shape. This is denoted as 1s2 where 1 is the level, s is the shape, and 2 is the number of electrons that fill this level.
The next level that will be filled would be 2s2. s implies the spherical or circular orbital only. Thus, this would be level 2, the shape s, and 2 electrons.
If the bilobed orbitals get filled at this level, then it would be referred to as 2p6. Here, the three bilobed orbitals would fill up, each carrying 2 electrons and giving a total of 6 electrons.
In this manner, d would have 5 different shapes (l = 2), each with 2 electrons giving d orbitals a maximum of 10 electrons. Finally, the f would have 7 different shapes (l = 3) each with 2 electrons giving f orbitals a maximum of 14 electrons.
The electron notation used allows all the atoms to be placed in a table, called the electron configuration table or the periodic table. Each part of the table represents the status of the different energy levels and orbitals.
For example, P for phosphorous. In order to write the electron notation, start at the top left and go to the right one line at a time. The notation would be 1s22s22p63s23p3. Since the P has only 3 electrons in the outer orbital, it can still acquire more electrons. The column to the far right is for noble gases, which are inert gases. These atoms have full outer orbitals. In addition, He is to the far right because the outer shell of s is full, with 2 electrons. It is colored blue since it belongs to the s orbital area.
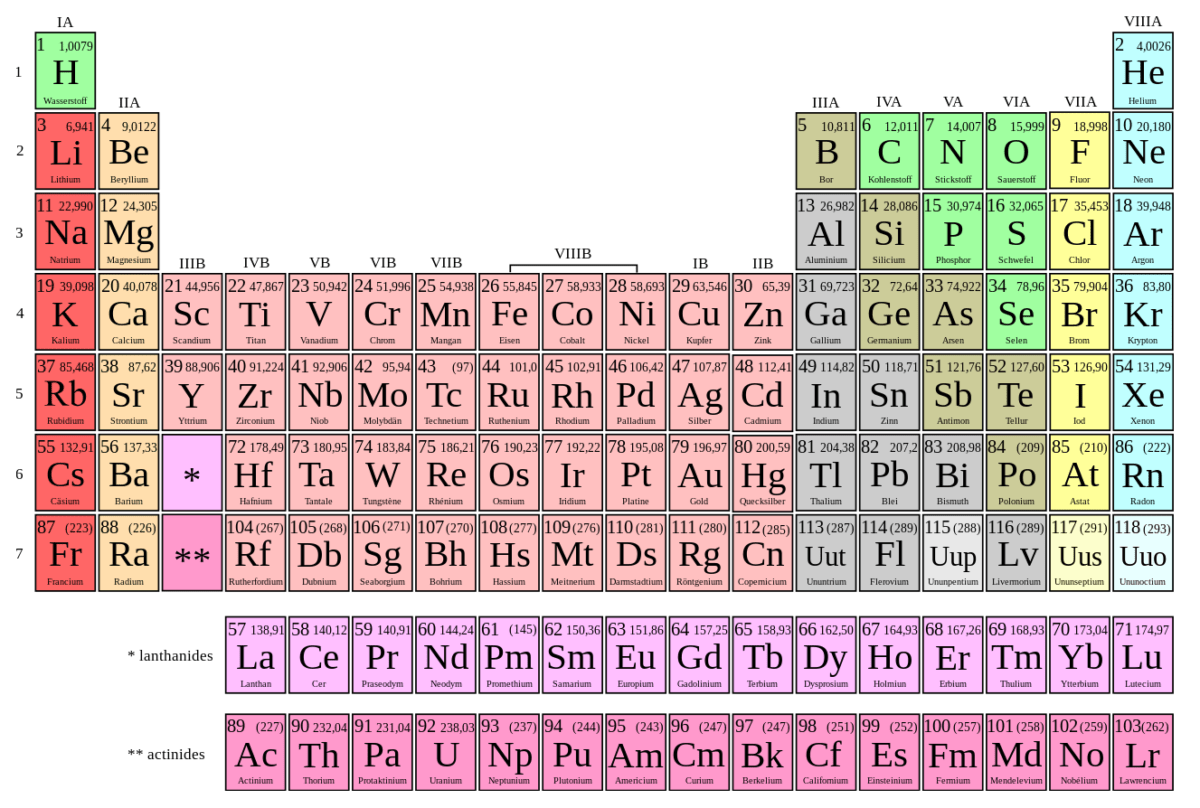
Periodic system of the elements
Image: “Periodic table : chemical symbols, atomic numbers, standard atomic weights and names in German” by Cdang. License: CC BY-SA 3.0Non-magnetic materials can respond to magnetic fields. If something is diamagnetic, electrons are usually paired, inducing a repulsive force. If something is paramagnetic, there usually is an unpaired electron, inducing an attractive force. As stated earlier, noble gases have full orbitals and paired electrons, so they are referred to as diamagnetic. Atoms and molecules can be attracted to magnets if they have unpaired electrons with a spin of the opposite side.
The effective nuclear charge is the net positive charge that an electron experiences. Outer electrons see an effectively weakened positive charge due to the shielding from inner electrons at lower levels. The outer shell electron experiences an effective nuclear charge, which is referred to as the core charge.
This is also known as the Heisenberg Uncertainty Principle or Indeterminacy Principle. Each particle exists at a position with a particular momentum. Unfortunately, neither of these measurements are very precise. Heisenberg stated that the uncertainty principle helps estimate the particle’s physical properties, in regards to position and momentum, mathematically. It is impossible, practically or theoretically, to measure the position and velocity of any object at the same time.
A particle’s position is described as the probability Probability Probability is a mathematical tool used to study randomness and provide predictions about the likelihood of something happening. There are several basic rules of probability that can be used to help determine the probability of multiple events happening together, separately, or sequentially. Basics of Probability of location with a wave A wave Cardiac Cycle. The momentum is related to the wavelength of the uncertainty wave. However, to narrow down the particle’s location, construct many waves with varying wavelengths and momentums. Adding the waves together creates a new waveform from constructive interference and destructive interference. The new wave is called the wave packet and shows more of a centralized location of the particle’s position and momentum, as seen in the figure below.
The top figure shows the uncertainty in momentum, whereas the bottom figure shows the uncertainty in the position. Both are inversely related; i.e., if uncertainty in momentum decreases, then the uncertainty in position increases, and vice versa.
The photoelectric effect is the release Release Release of a virus from the host cell following virus assembly and maturation. Egress can occur by host cell lysis, exocytosis, or budding through the plasma membrane. Virology, or emission, of electrons when light is shone onto an object, material, or atom. The higher the intensity of light radiated, the more excited the electrons become, and the more violent/speedy is the emission. Light can entirely release Release Release of a virus from the host cell following virus assembly and maturation. Egress can occur by host cell lysis, exocytosis, or budding through the plasma membrane. Virology electrons from their orbitals. However, only high-frequency light causes electrons to be released. When the red light is used, no electrons are released. When higher energy blue light is used, electrons are able to be released.
The energy of release Release Release of a virus from the host cell following virus assembly and maturation. Egress can occur by host cell lysis, exocytosis, or budding through the plasma membrane. Virology is called the work function ( phi PHI Information generated while providing medical care that can be used to identify a patient. Patient-Doctor Confidentiality). The photon’s energy partially contributes to releasing the electron and partially to the released electron’s kinetic energy.