Chemical bonding refers to the process of combining two or more atoms to form a molecule or compound. Intramolecular bonds are strong bonds that hold atoms together within a molecule and may be ionic, covalent, or metallic. Ionic bonding occurs when electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. Covalent bonding occurs when more atoms share electrons. Sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Metallic bonding involves the sharing of electrons among a lattice of positively charged metal ions. Intermolecular bonds are weak and include Van der Waals bonds, which are weak intermolecular forces that arise due to the temporary dipoles created by the movement of electrons in a molecule. Hydrogen bonds are special types of dipole-dipole interactions that are stronger than Van der Waals forces.
Last updated: Mar 31, 2023
Ionic bonding is a type of chemical bond that is based on the force of attraction between oppositely-charged ions (Coulomb-force, electrostatic force). Due to the ionic bond, there occurs a regular Regular Insulin arrangement of ions, which is also called ionic crystal. If a solid is built up by ions, then it is considered a salt. They are weaker and less stable but still abide by the octet rule. Common table salt (NaCl) is formed by ionic bonds.
The following are the common characteristics of an ionic crystal:
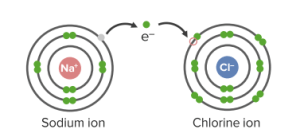
Ionic bonds in NaCl
Image by Lecturio.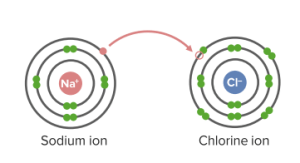
Ionic bonds in NaCl
Image by Lecturio.An atomic bond is a type of chemical bond that is based on the formation of a common electron pair. The atoms have solid partners, referring to a directed binding. Single covalent bonds involve the sharing of one pair of electrons. Hydrogen has a valence of 1 & 1 valence electron; 2 electrons in the valence shell will satisfy the octet rule. Each has 2 electrons in the valence shell.
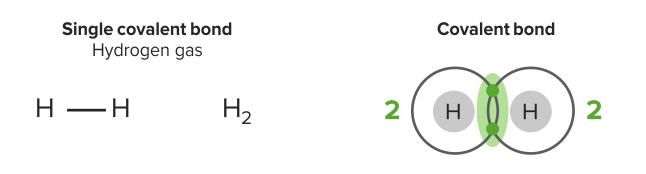
Single covalent bond
Image by Lecturio.Double covalent bonds involve the sharing of two pairs of electrons (stronger). Oxygen has 6 valence electrons. It needs 2 more electrons to satisfy the octet rule. It shares 2 electrons with another oxygen. They have 2 pairs of shared electrons in this bond. The substance class of molecular substances can be derived from this type of binding.
Substance properties: Relatively low melting and boiling temperature.
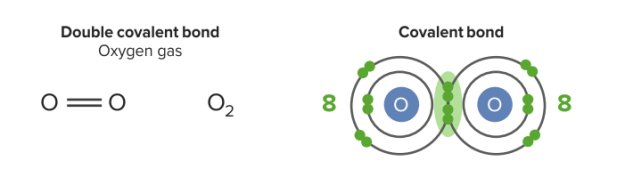
Double covalent bond:
They have 2 pairs of shared electrons in this bond. Each oxygen has 8 electrons in the valence shell (stable).
The metallic bond is a type of chemical bond that is based on the forces of attraction between positively charged metal ions and negatively charged, versatile ions. The substance class can be derived from this binding type.
Characteristics: regular Regular Insulin, a grid-like arrangement of the positively charged metal ions in a space
Substance properties:
Calculation: Not calculable, as it occurs in metals and alloys
Hydrogen bonds form between polar molecules:
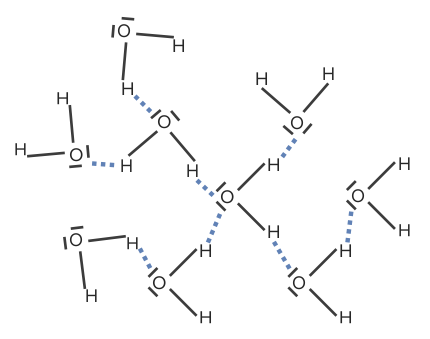
Hydrogen bonds
Image by Lecturio.The Van-der-Waals forces represent weak forces of attraction or non-covalent interactions between molecules, atoms or ions. It is dependent on the particular size and the contact surface.
If two molecules interact via a hydrogen atom, then so-called hydrogen bridges occur. Strong forces of attraction are formed between the positively charged hydrogen atom, and a free electron pair of a nitrogen Nitrogen An element with the atomic symbol n, atomic number 7, and atomic weight [14. 00643; 14. 00728]. Nitrogen exists as a diatomic gas and makes up about 78% of the earth’s atmosphere by volume. It is a constituent of proteins and nucleic acids and found in all living cells. Urea Cycle, oxygen, or fluorine atom. Hydrogen bridges are only formed with the most electronegative elements (N, O, and F). At this, there is a donator and an acceptor.
In the case of the donor, the hydrogen atom is bonded to a highly electronegative partner, whereby the hydrogen atom becomes the positive pole (positive partial charge) and the binding partner becomes the negative pole. The acceptors are generally covalently bonded nitrogen Nitrogen An element with the atomic symbol n, atomic number 7, and atomic weight [14. 00643; 14. 00728]. Nitrogen exists as a diatomic gas and makes up about 78% of the earth’s atmosphere by volume. It is a constituent of proteins and nucleic acids and found in all living cells. Urea Cycle, oxygen, or fluorine atoms, which possess a negative partial charge.
The relevance of the hydrogen bridges in the biochemistry:
Sigma bonds are types of bonds in a molecular structure that are formed by end-to-end overlap of atomic orbitals. Unless this type of overlap is possible, a sigma bond may not form. One simple sigma bond is the one present in an H2 molecule. Since an H atom only has an s-orbital, the overlap will be between two s-orbitals.
Overlap between two p-orbitals is also possible. To form a sigma bond, the interaction should be a head-to-tail interaction, which is one lobe of one p-orbital faces one lobe of the other p-orbital, enabling the overlap as in the figure below.
Sigma bonds may also be formed by the interaction of dissimilar orbitals. In the case of the bond between Hydrogen and Fluorine in hydrogen fluoride Fluoride Inorganic salts of hydrofluoric acid, hf, in which the fluorine atom is in the -1 oxidation state. Sodium and stannous salts are commonly used in dentifrices. Trace Elements, the H atom only has an s-orbital available for bonding, while the F atom has a p-orbital. Even though the two orbitals are different, a sigma bond may still be formed as long as the two interacting orbitals are in the correct orientation Orientation Awareness of oneself in relation to time, place and person. Psychiatric Assessment. In this way, sharing of electrons will be achieved between the two atoms.
For example, if the s and p-orbital interacting with each other are in an orientation Orientation Awareness of oneself in relation to time, place and person. Psychiatric Assessment where one of the lobes of the p-orbital is directly facing the s-orbital, a sigma bond may form because overlap of orbitals is possible. If the s-orbital of the H atom is not directly facing any of the two lobes of the p-orbital, no sigma bond will be formed because orbital overlap will not occur.
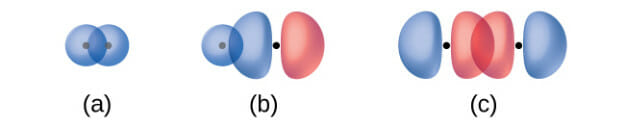
Sigma bonds:
Sigma (σ) bonds form from the overlap of the following: (a) two s orbitals, (b) an s orbital and a p orbital, and (c) two p orbitals. The dots indicate the locations of the nuclei.
For heteroatomic molecules, the overlap of simple s and p-orbitals will result in a highly restricted molecular structure. For example, the compound methane does not follow an ordinary square planar structure; instead, it follows a tetrahedral configuration. Also, since the p-orbitals are oriented in different planes, this would involve different energy values. To aid this, hybridization Hybridization The genetic process of crossbreeding between genetically dissimilar parents to produce a hybrid. Blotting Techniques should occur. Orbital hybridization Hybridization The genetic process of crossbreeding between genetically dissimilar parents to produce a hybrid. Blotting Techniques is the mixing of atomic orbitals into new hybrid orbitals, with different shapes and energies to the component atomic orbital, suitable for electron pairing during the formation of covalent bonds.
In a methane molecule, it is not possible to form four sigma bonds due to 1 s-s and 3 s-p interaction. This is because they will have different amounts of energy involved. What happens then is the one s and the 3 p-orbitals of the C atom will be hybridized to form 4 sp3 hybrid orbitals that are all similar in energies and shapes. Each of these sp3-orbitals will overlap with the s-orbital of an H atom forming four sigma bonds, resulting in a tetrahedral configuration.
Different types of hybrid orbitals may be formed, depending on the type of atomic orbitals that combine. If one s and one p-orbital are mixed, two sp hybrid orbitals are formed. On the other hand Hand The hand constitutes the distal part of the upper limb and provides the fine, precise movements needed in activities of daily living. It consists of 5 metacarpal bones and 14 phalanges, as well as numerous muscles innervated by the median and ulnar nerves. Hand: Anatomy, if one s and two p orbitals are mixed, three sp2-orbitals are formed. The number of hybrid orbitals corresponds to the number of sigma bonds that the atom can form. For example, if an atom has two sp hybridized orbitals, this means it can form two sigma bonds. The same case is if an atom has three sp2 hybridized orbitals, three sigma bonds can be formed.
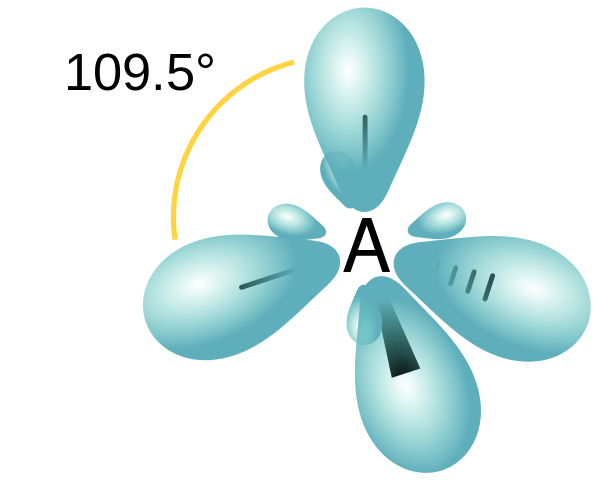
Orbital hybridisation sp3
Image: “Orbital hybridisation sp3” by Jfmelero. License: CC BY-SA 3.0Between two atoms, only one single bond is possible to be formed. This is because there is only one way for end-to-end interaction to occur, especially since the p orbitals are oriented in different planes. Another type of orbital overlap is possible. If two p-orbitals are oriented parallel to each other, a side-by-side overlap is possible. This type of interaction leads to the formation of pi bonds.
Pi bonds are weaker than sigma bonds because of the weaker overlap between the orbitals. Electrons in pi bonds can also be referred to as pi electrons. Pi bonds are present in multiple bonds (double or triple bonds). Pi interaction is only possible between atoms that are already sigma bonded.
In the case of the compound ethene, three sigma bonds are formed by each of the C atoms. This means that 3 hybrid orbitals were used. This is only possible if 1 s and 2p-orbitals have undergone hybridization Hybridization The genetic process of crossbreeding between genetically dissimilar parents to produce a hybrid. Blotting Techniques. This means each of the C atoms will have an extra p-orbital that can overlap with the p-orbital of the other C atom to form a pi bond.
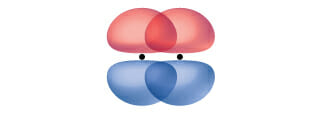
Pi bonds:
Pi (π) bonds form from the side-by-side overlap of two p-orbitals. The dots indicate the location of the nuclei.
Double bonds and Triple bonds only differ in the number of pi bonds present in the bond. A double bond will contain one sigma bond and one pi bond, while a triple bond contains one sigma bond and two pi bonds.
There is a correlation Correlation Determination of whether or not two variables are correlated. This means to study whether an increase or decrease in one variable corresponds to an increase or decrease in the other variable. Causality, Validity, and Reliability between the type of hybrid orbital and the type of bond present around an atom. A carbon compound with the C atom containing 4 sp3-orbitals can form 4 sigma bonds. A carbon compound with the C atom containing 3 sp2-orbitals and one p can form 3 sigma bonds and 1 pi bond. A carbon with the C atom containing 2 sp-orbitals and 2 p-orbitals can form 2 sigma bonds and 2 pi bonds. This leads to the formation of double and triple bonds.