Thermochemistry is a branch of thermodynamics that deals specifically with the study of the heat Heat Inflammation energy associated with chemical reactions. Enthalpy Enthalpy Enzyme Kinetics is the sum of the internal energy of a body or system and the product of its volume multiplied by the pressure, and entropy Entropy The measure of that part of the heat or energy of a system which is not available to perform work. Entropy increases in all natural (spontaneous and irreversible) processes. Enzyme Kinetics is the energy of a closed system that is unavailable to do work. Hess's Law is a principle in thermochemistry that states that the enthalpy Enthalpy Enzyme Kinetics change for a chemical reaction is the same whether the reaction takes place in one step or in a series of steps. Gibbs Free Energy Free energy Enzyme Kinetics is a measure of the thermodynamic potential of a system to do work. It is the energy that can be used to do useful work. The coefficient of thermal expansion is a measure of how much a material expands or contracts when it is heated or cooled.
Last updated: Mar 31, 2023
Hess’s law of constant heat Heat Inflammation summation, or Hess’s law, states that no matter how many steps or stages are present in a reaction, the total enthalpy Enthalpy Enzyme Kinetics change for the entire reaction is the sum of each individual change. Enthalpy Enthalpy Enzyme Kinetics is a thermodynamic measurement assessing the total heat Heat Inflammation content of a system (see image below). It is equivalent to the internal energy of the system plus the product of the pressure and volume:
H = U + pV PV Polycythemia vera (PV) is a chronic myeloproliferative neoplasm characterized by the overproduction of rbcs. In addition, the wbc and platelet counts are also increased, which differentiate pv from erythrocytosis seen with chronic hypoxia and other chronic conditions. Polycythemia Vera
Where:
H = enthalpy Enthalpy Enzyme Kinetics
U = internal energy
p = pressure
V = volume
In the reaction A → B → C, substance A is undergoing a reaction to become B, which then undergoes another reaction to become C. The change in enthalpy Enthalpy Enzyme Kinetics of the energy change is denoted by ΔH.
The ΔH for the total reaction is equal to the sum of the ΔH for the first reaction, plus the ΔH for the second reaction:
ΔHA͢͢͢͢͢ to B + ΔHB to C = ΔHA to C
Example of the calculation that takes place in a reaction:
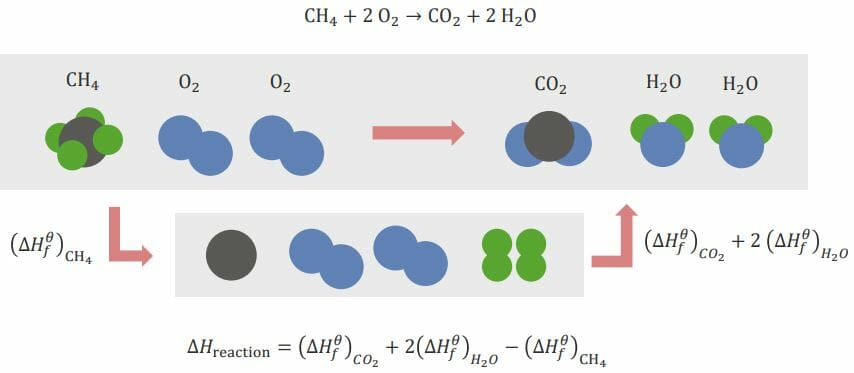
CH4 + 2O2 → CO2 + 2H2O
Here, methane and oxygen react to form carbon dioxide and water. The elements need to be rearranged before the reaction can occur. This is done by breaking existing bonds and forming new bonds between elements.
CH4 (methane) needs to be broken down into its simple elements: C (carbon) and H (hydrogen). When this happens, an energy change occurs; this is referred to as (ΔHf)CH4. This is the standard energy of formation referring to standard elements present in nature. O2 (oxygen) does not need to be broken down into individual O elements since, in nature, a single O isn’t present. The standard form is O2.
To form the products, C and O have to combine to form CO2 (carbon dioxide) and H and O have to combine to form H2O (water). The enthalpies for forming these two products are (ΔHf)CO2 and (ΔHf)H2O, respectively.
The total enthalpy Enthalpy Enzyme Kinetics of the entire reaction is as follows:
ΔHreaction = (ΔHf)CO2 + 2(ΔHf)H2O – (ΔHf)CH4
The minus sign for the enthalpy Enthalpy Enzyme Kinetics for methane is due to the break-up of the bonds that are taking place:
Hess’s-law
Image by Lecturio.Hess’s law is used to:
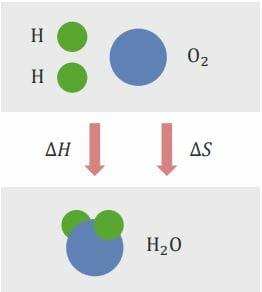
Gibbs free energy Free energy Enzyme Kinetics is the energy that is associated with a chemical reaction that can be used to perform work. It conveys the amount of free energy Free energy Enzyme Kinetics that exists for a reaction to go forward (see image below). In general, a reaction wants to minimize energy use ( enthalpy Enthalpy Enzyme Kinetics) and maximize entropy Entropy The measure of that part of the heat or energy of a system which is not available to perform work. Entropy increases in all natural (spontaneous and irreversible) processes. Enzyme Kinetics.
Entropy Entropy The measure of that part of the heat or energy of a system which is not available to perform work. Entropy increases in all natural (spontaneous and irreversible) processes. Enzyme Kinetics is the degree of disorder or randomness that exists in a system. The calculation of Gibbs free energy Free energy Enzyme Kinetics (ΔG) determines whether the reaction can occur spontaneously or not.
Enthalpy Enthalpy Enzyme Kinetics change (ΔH) and entropy Entropy The measure of that part of the heat or energy of a system which is not available to perform work. Entropy increases in all natural (spontaneous and irreversible) processes. Enzyme Kinetics change (ΔS) are competing reactions. Enthalpy Enthalpy Enzyme Kinetics seeks to be minimal, whereas entropy Entropy The measure of that part of the heat or energy of a system which is not available to perform work. Entropy increases in all natural (spontaneous and irreversible) processes. Enzyme Kinetics seeks to be maximal.
Entropy Entropy The measure of that part of the heat or energy of a system which is not available to perform work. Entropy increases in all natural (spontaneous and irreversible) processes. Enzyme Kinetics is also associated with temperature, since the disorder that exists in a system is dependent on temperature. The higher the temperature, the more disorder is present.
The relationship between Gibbs free energy Free energy Enzyme Kinetics (ΔG), enthalpy Enthalpy Enzyme Kinetics change (ΔH), and entropy Entropy The measure of that part of the heat or energy of a system which is not available to perform work. Entropy increases in all natural (spontaneous and irreversible) processes. Enzyme Kinetics change (ΔS) is as follows:
ΔG = ΔH – T ΔS
Where :
ΔG = Gibbs free energy Free energy Enzyme Kinetics
H = enthalpy Enthalpy Enzyme Kinetics
TS = random energy
ΔGsystem = –T ΔS total
Note the negative sign above; it denotes that enthalpy Enthalpy Enzyme Kinetics and entropy Entropy The measure of that part of the heat or energy of a system which is not available to perform work. Entropy increases in all natural (spontaneous and irreversible) processes. Enzyme Kinetics are opposing reaction concepts; as well, temperature (T) is measured using the Kelvin scale Scale Dermatologic Examination (K).
To Be Spontaneous or Not To Be Spontaneous; That is the Question
The reaction that needs to take place can occur either spontaneously (favorably) or nonspontaneously (unfavorably). This is determined by the calculation of Gibbs free energy Free energy Enzyme Kinetics (ΔG).
If ΔG < 0, then the reaction is considered to be a spontaneous reaction. If ΔG > 0, then the reaction is considered to be a non-spontaneous reaction.
Even if the reaction is exothermic (i.e., it requires no energy input), it may not occur if it lowers the entropy Entropy The measure of that part of the heat or energy of a system which is not available to perform work. Entropy increases in all natural (spontaneous and irreversible) processes. Enzyme Kinetics too much. As well, even if something is spontaneous, it does not necessarily occur quickly. Both of these concepts are not directly related, since they depend on different properties of the reaction.
Gibbs free energy
Image by Lecturio.The coefficient of thermal expansion describes how the size of different types of matter can be affected (changed) by a change in temperature. Specifically, it measures the fractional change in size per degree change in temperature at a constant pressure. This change can be associated with linear (one-dimensional) change or volumetric (three-dimensional) change. When an object is heated (i.e., its temperature increases), the heated object is larger. Increasing heat Heat Inflammation will continue causing some expansion to occur based on the degree of Kelvin change in temperature.
An object has a linear dimension which, when heated, undergoes a linear expansion. The linear thermal expansion can only be measured in the solid state and is common in engineering applications. This change in thermal expansion in the linear (one-dimensional) direction is denoted as follows:
Lfinal = Linitial (1 + α ΔT)
Where:
Lfinal = final length
Linitial = initial length
α = coefficient of linear expansion
ΔT = temperature change
The coefficient of linear expansion is α = 1 / temperature; thus, the units for α are 1 / Kelvin.
An object has a volumetric dimension that, when heated, undergoes volumetric expansion. The volumetric thermal expansion can be measured for all condensed matter (liquid and solid states). This change in thermal expansion in the volumetric (three-dimensional) direction is denoted as follows:
Vfinal = Vinitial (1 + αv ΔT)
Where:
Vfinal = final volume
Vinitial = initial volume
αv = coefficient of volumetric expansion
ΔT = temperature change
The coefficient of volumetric expansion is αv = 3 α. This is because the volumetric coefficient is associated with three dimensions; thus, it is equal to three of the coefficient of linear expansions.
The thermal expansion equations for linear expansion and volumetric expansion can be stated in a different way based on a change in length and volume, respectively.
Lfinal = Linitial (1 + α ΔT)
Lfinal = Linitial + (Linitial α ΔT)
Lfinal = Linitial + ΔL
Thus, the change in length is ΔL = Linitial α ΔT.
Vfinal = Vinitial (1 + αv ΔT)
Vfinal = Vinitial + (Linitial αv ΔT)
Vfinal = Vinitial + ΔL
Thus, the change in volume is ΔV = Vinitial αv ΔT.
Applications of thermal expansion include the following: