Enzyme inhibitors bind BIND Hyperbilirubinemia of the Newborn to enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body's constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes and decrease their activity. Enzyme activators bind BIND Hyperbilirubinemia of the Newborn to enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body's constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes and increase their activity. Molecules that decrease the catalytic activity of enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body's constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes can come in various forms, including reversible or irreversible inhibition. Reversible inhibition can be competitive, non-competitive, or uncompetitive.
Last updated: May 12, 2025
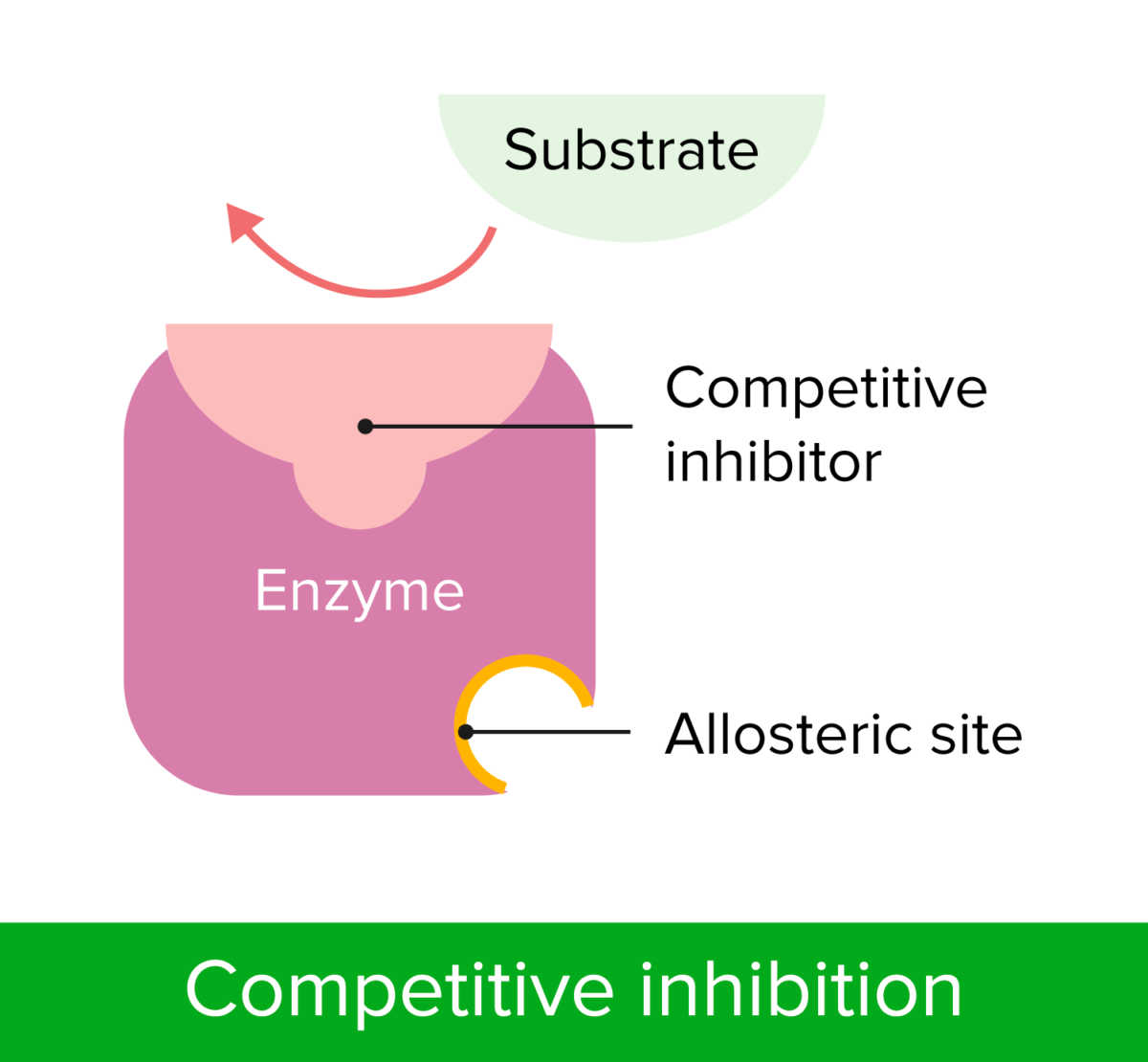
Competitive inhibition
Image by Lecturio.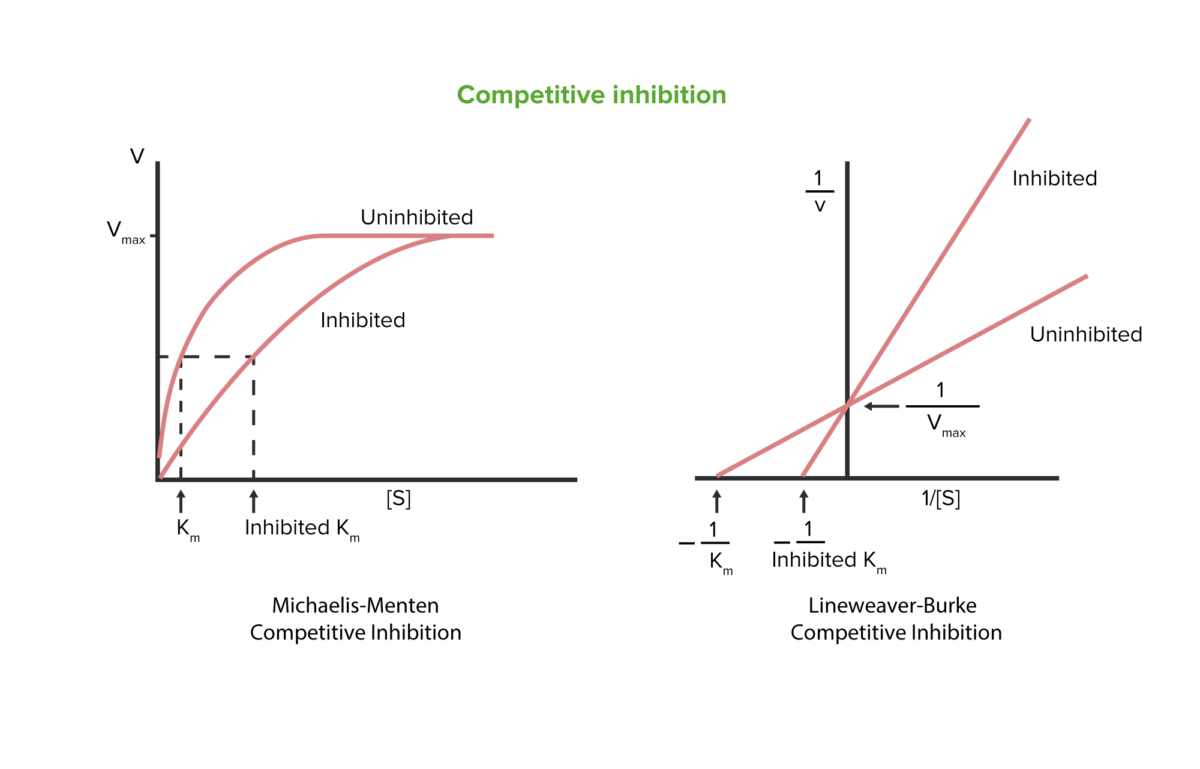
Competitive inhibition
Image by Lecturio.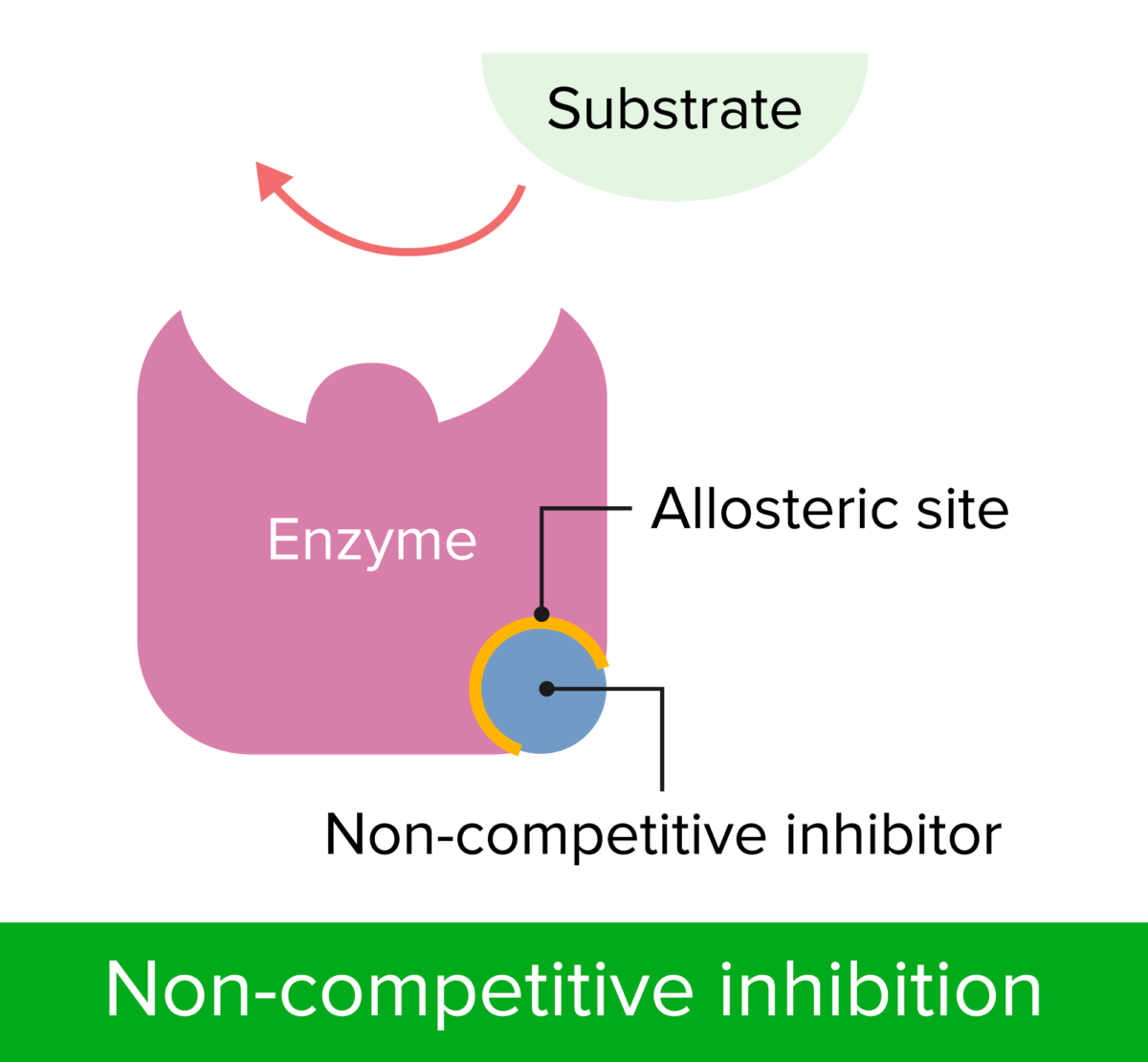
Non-competitive inhibition
Image by Lecturio.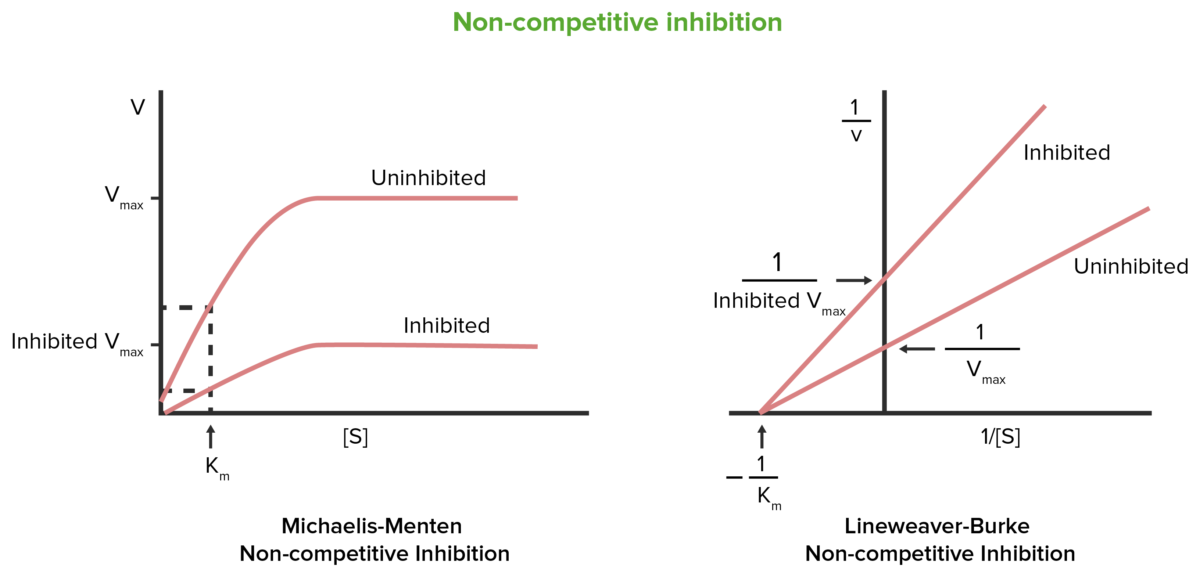
Non-competitive inhibition
Image by Lecturio.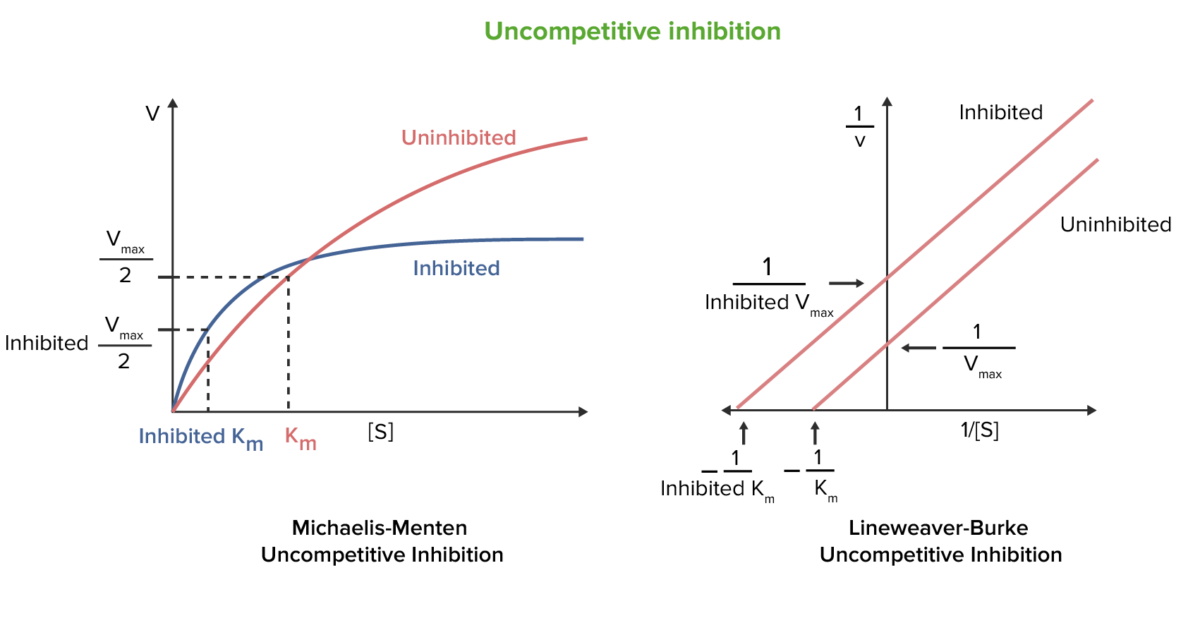
Uncompetitive reversible enzyme inhibition
Image by Lecturio.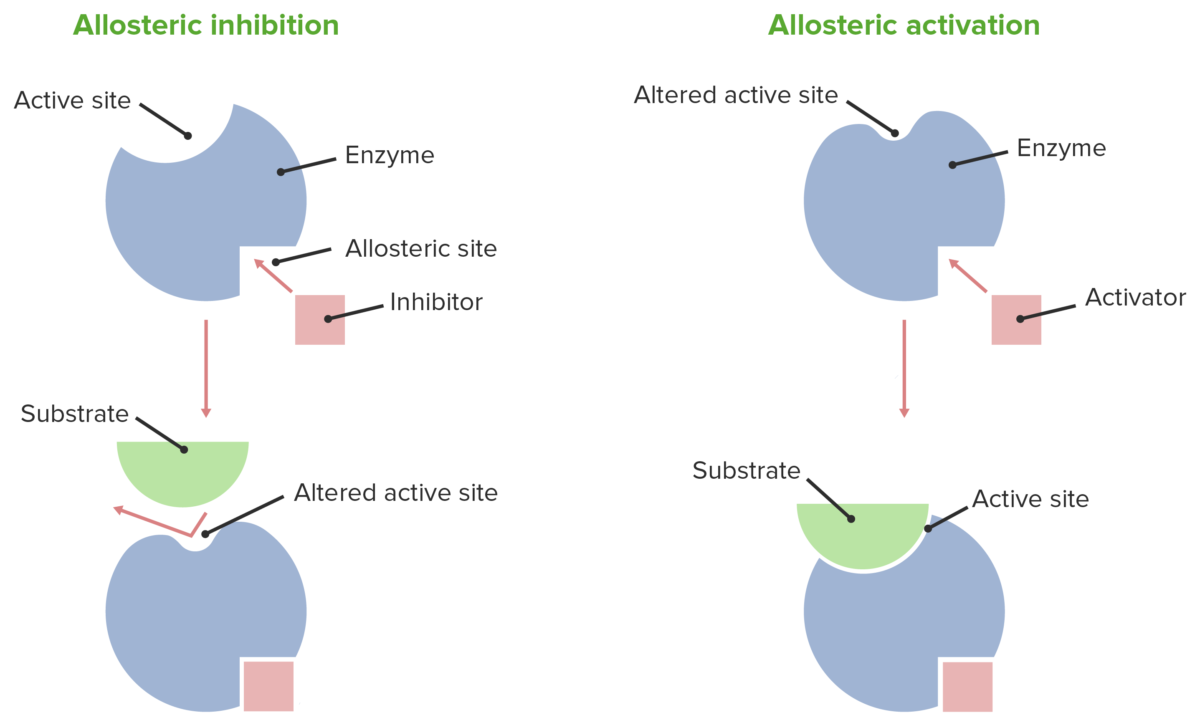
Allosteric effects
Image by Lecturio.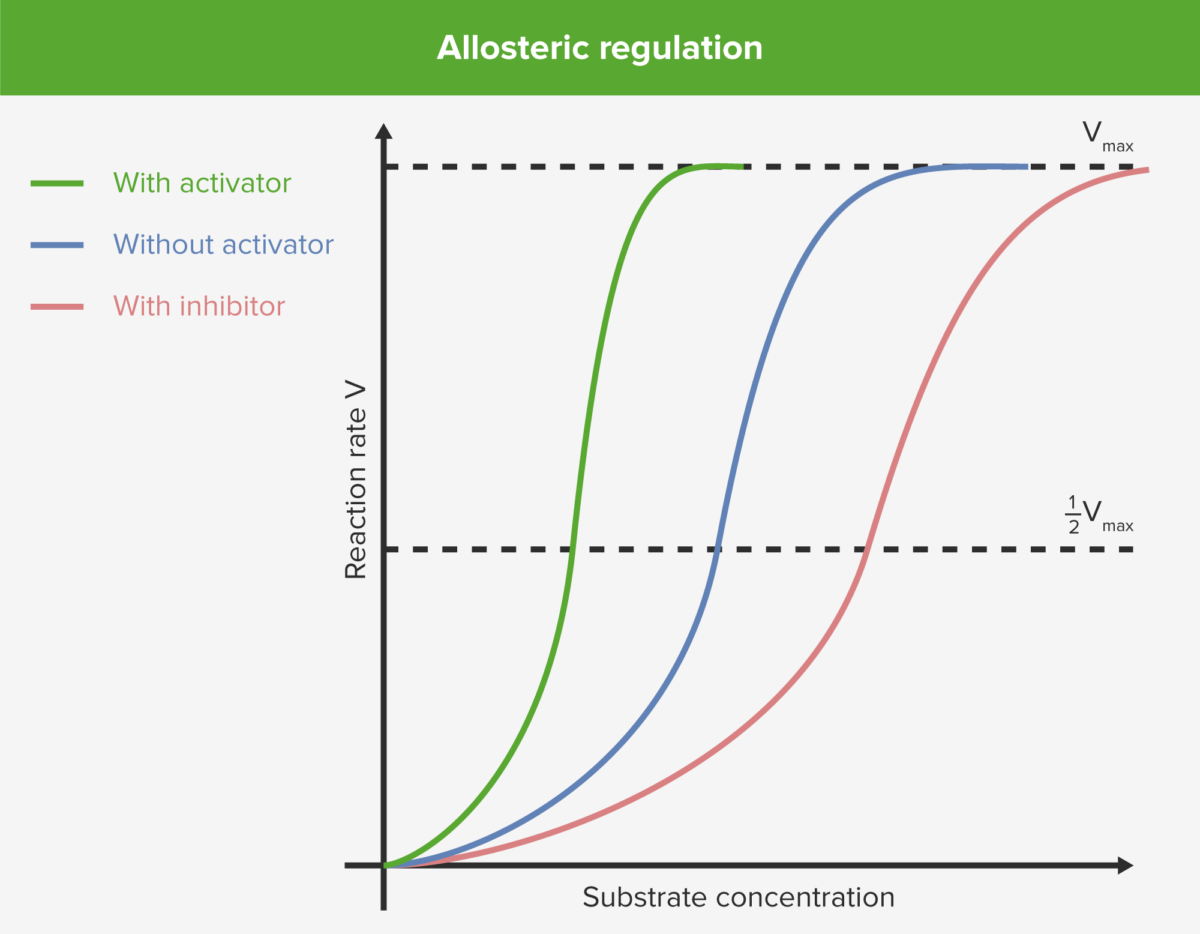
Allosteric regulation
Image by Lecturio.