Epidemiological studies are used to establish associations between risk factors and health-related outcomes. These studies can take the form of observational or interventional studies. Observational studies are nonexperimental investigations of the associations between known exposures and outcomes. Interventional studies are designed to evaluate the direct impacts of a risk factor on a disease by applying an intervention to the subjects in an experimental group and prospectively comparing the effects with a control group. Interventional studies are more expensive and time-consuming but tend to provide stronger evidence for an association than observational studies. Depending on the situation, investigators will typically choose a combination of these study designs.
Last updated: Oct 30, 2024
Observational studies are used to observe and measure outcomes in a cohort with no control over risk factors or variables. They are often retrospective. Types of observational studies include cross-sectional studies, case-control studies, and cohort studies.
| Advantages | Disadvantages |
|---|---|
| Direct calculation of incidence Incidence The number of new cases of a given disease during a given period in a specified population. It also is used for the rate at which new events occur in a defined population. It is differentiated from prevalence, which refers to all cases in the population at a given time. Measures of Disease Frequency rates | Time-consuming |
| May yield information on the incidence Incidence The number of new cases of a given disease during a given period in a specified population. It also is used for the rate at which new events occur in a defined population. It is differentiated from prevalence, which refers to all cases in the population at a given time. Measures of Disease Frequency of disease | Often requires a large sample size Sample size The number of units (persons, animals, patients, specified circumstances, etc.) in a population to be studied. The sample size should be big enough to have a high likelihood of detecting a true difference between two groups. Statistical Power |
| Clear temporal relationship between exposure and disease | Expensive |
| Particularly efficient for the study of rare exposures | Not efficient for the study of rare diseases |
| Can yield information on multiple exposures | Losses to follow-up may diminish their validity Validity Validity refers to how accurate a test or research finding is. Causality, Validity, and Reliability |
| Can yield information on multiple outcomes of a particular exposure | Changes over time in diagnostic methods may lead to biased results |
| Minimizes bias Bias Epidemiological studies are designed to evaluate a hypothesized relationship between an exposure and an outcome; however, the existence and/or magnitude of these relationships may be erroneously affected by the design and execution of the study itself or by conscious or unconscious errors perpetrated by the investigators or the subjects. These systematic errors are called biases. Types of Biases |
| Exposure | Outcome | Study type |
|---|---|---|
| Uncommon | Common | Cohort |
| Common | Uncommon | Case-control |
| Scenario | Study type | |
| Short-term study of current, uncommon exposures and common outcomes | Prospective cohort | |
| Short- or long-term study of historic, uncommon exposures and common outcome | Retrospective cohort | |
| Outbreak investigation | Case-control | |
| Instantaneous survey, common outcomes and no change over time | Cross-sectional | |
| Is there an association between climbing Mount Everest and getting diabetes Diabetes Diabetes mellitus (DM) is a metabolic disease characterized by hyperglycemia and dysfunction of the regulation of glucose metabolism by insulin. Type 1 DM is diagnosed mostly in children and young adults as the result of autoimmune destruction of β cells in the pancreas and the resulting lack of insulin. Type 2 DM has a significant association with obesity and is characterized by insulin resistance. Diabetes Mellitus? | Cohort | |
| Is there an association between left-handedness and getting mad cow disease? | Case-control | |
| Is there an association between left-handedness and gender Gender Gender Dysphoria? | Cross-sectional | |
| What factor was likely responsible for the salmonella Salmonella Salmonellae are gram-negative bacilli of the family Enterobacteriaceae. Salmonellae are flagellated, non-lactose-fermenting, and hydrogen sulfide-producing microbes. Salmonella enterica, the most common disease-causing species in humans, is further classified based on serotype as typhoidal (S. typhi and paratyphi) and nontyphoidal (S. enteritidis and typhimurium). Salmonella outbreak at the office holiday party? | Case-control | |
| Was there an association between working on the nuclear bomb project in World War II and developing cancer 5 years later? | Retrospective cohort | |
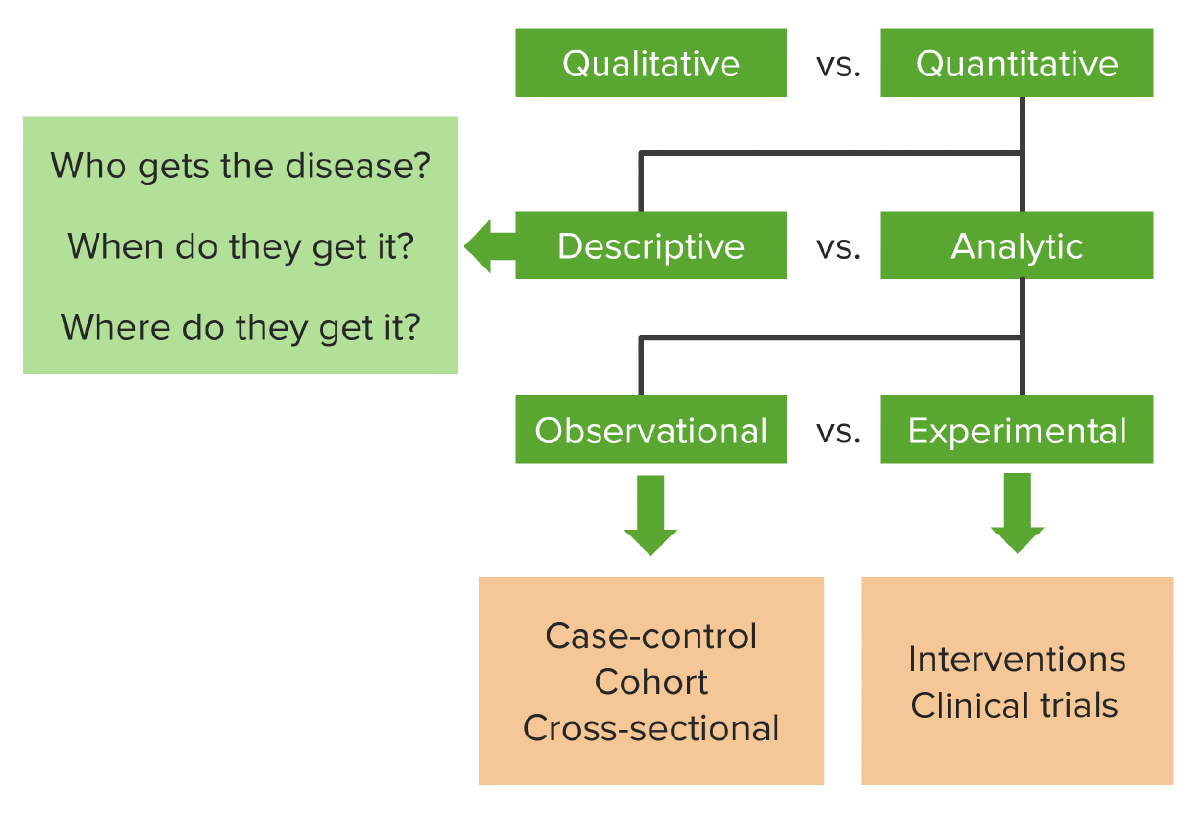
A flowchart of the different types of research (observational vs. experimental) and their representative studies. Observational studies passively look at a cohort with a disease or condition over a period of time (often retrospectively); experimental studies prospectively look at the effect of a particular intervention or exposure in a group as compared to controls.
Image by Lecturio.Interventional studies are prospective experimental trials in which investigators compare the effects of an intervention on subjects with a control group. The most compelling type of interventional study is the RCT.
| Advantages | Disadvantages |
|---|---|
| Confounding variables Confounding variables A confound is an additional variable other than the independent variable that has an effect on the dependent variable, causing an erroneous relationship to be inferred between them. Types of Biases are well-controlled | Cost |
| Temporal relationship is well-established | Study groups do not necessarily represent the real world. |
| If blinded, provides strong evidence for causation | Ethically problematic |
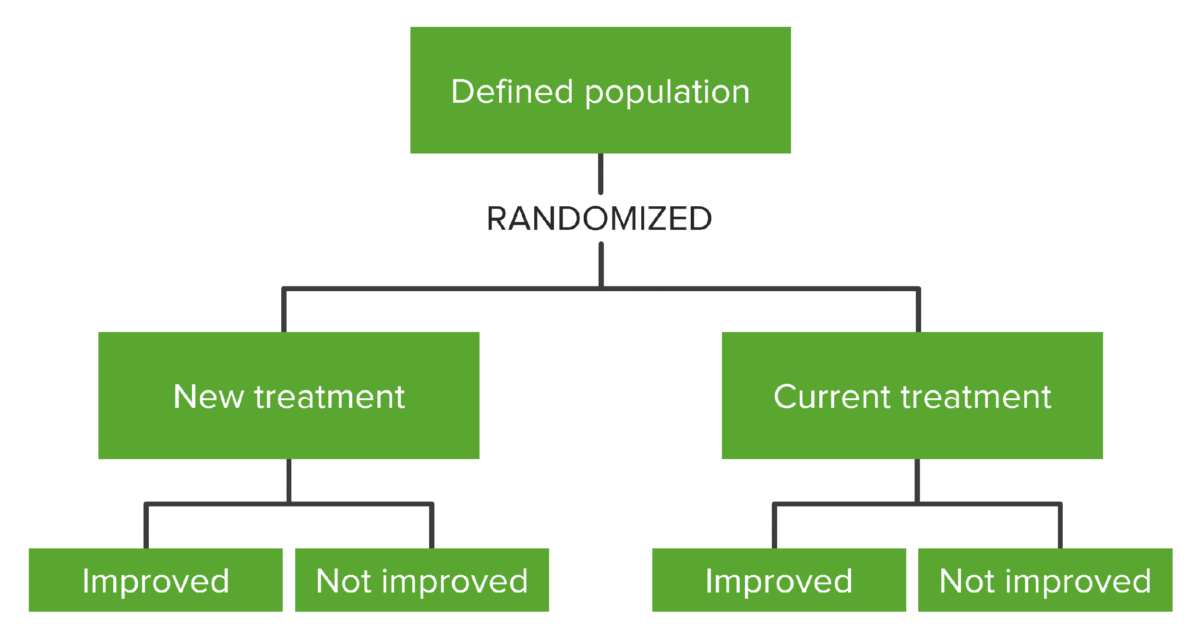
Flowchart showing how a defined population is distributed into different study groups through randomization when setting up an RCT. Randomization is carried out by the investigators so participants don’t choose which group they’ll be a part of.
Image by Lecturio.| RCTs | Case-control studies |
|---|---|
| Prospective | Retrospective/prospective |
| Experimental study: An intervention (exposure) (e.g., drug, screening Screening Preoperative Care test) is applied to a group and effects are compared to a control group. | Observational study: A group is chosen with a common exposure and followed longitudinally to track to development of an outcome (e.g., disease, condition). |
| Subjects are randomized by investigators before exposure occurs. | The subjects, or medical records, report their exposure. |
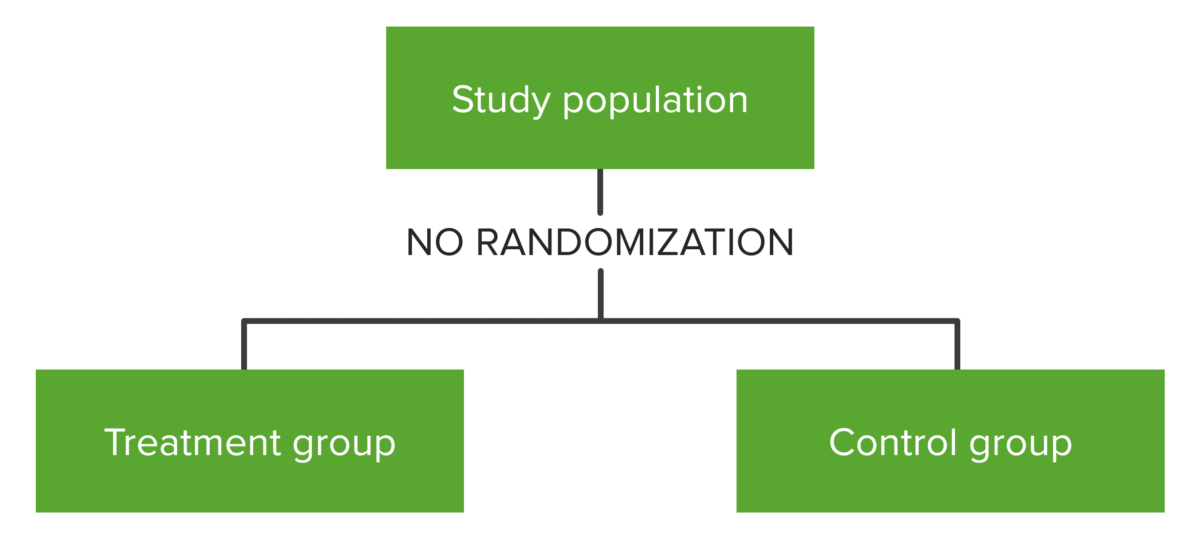
Conceptual map showing the distribution of the subjects in studies that employ a quasi-experimental design. Note the lack of randomization.
Image by Lecturio.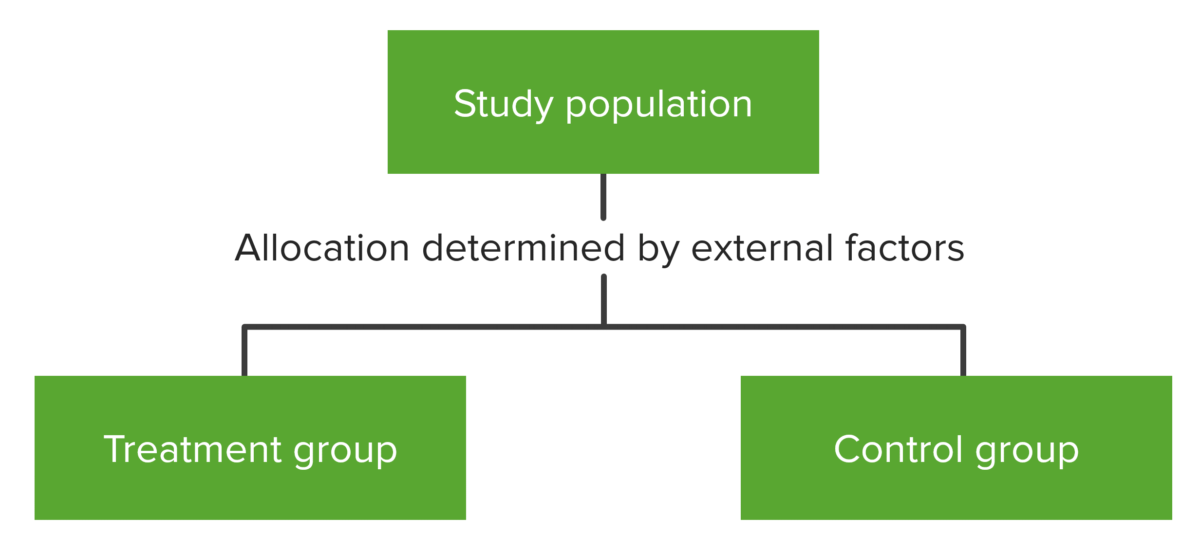
Conceptual map showing the distribution of the study population in natural experiments. Randomization is not carried out by the investigators but by an external force and is subject to bias.
Image by Lecturio.