Stereochemistry is the branch of science which studies all aspects of the three-dimensional shapes of molecules. “Stereo” is Latin for "three-dimensional." This article provides a fundamental explanation of the following: basic principles, terminologies, and biological significances of stereochemistry and chirality in real life.
Last updated: Nov 3, 2022
Jean-Baptiste Biot first discovered the optical activity Optical activity Optical activity is the ability of stereoisomers to rotate plane-polarized light. The molecules are designated dextrorotatory (+) or levorotatory (-) depending on the direction of light rotation Basics of Carbohydrates of molecules in 1815. In 1848, Louis Pasteur, who is credited as being the first stereochemist, observed that tartaric acid salts could rotate plane polarized light (i.e., be optically active), while other salts from other sources could not. In 1874, Jacobus H. Vant Hoff and Joseph A. LeBel proposed the Theory of Organic Structure in Three-Dimensions which theorized that:
Molecular structures are categorized as symmetrical Symmetrical Dermatologic Examination, dissymmetrical, or asymmetrical. The molecular structure is said to be symmetrical Symmetrical Dermatologic Examination when it displays the elements of symmetry. For example, the derived symmetry operation transforms it into a molecule, which is then superimposable.
| Symmetry elements | Symmetry operations |
|---|---|
| Proper (simple) axes of rotation Rotation Motion of an object in which either one or more points on a line are fixed. It is also the motion of a particle about a fixed point. X-rays | When a molecule rotates 360ᴼ around a symmetry axis (Cn n > 1″), and its arrangement cannot be distinguished from the original one. |
| Planes of symmetry | IWhen a mirror plane passes through the molecule and divides it into two symmetrical Symmetrical Dermatologic Examination halves, the reflection of all the atoms through this plane creates an arrangement that cannot be distinguished from the original one. |
| Center of symmetry (of inversion) | Every atom has a symmetrical Symmetrical Dermatologic Examination counterpart to this center. An inversion of all atoms relative to the center results in a molecule that is indistinguishable from the original one. |
| Rotation-reflection axes (mirror axes, improper axes, alternating axes) | If the molecule has an axis of rotation-reflection in which there is a 360ᴼ rotation Rotation Motion of an object in which either one or more points on a line are fixed. It is also the motion of a particle about a fixed point. X-rays around this axis, followed by reflection through a mirror plane thereby giving a superimposable image, it is said to display reflection symmetry. |
Molecular structures without reflection symmetry (no plane of symmetry) are called: dissymmetric or chiral. If a symmetry axis Cn (n > 1) is also absent, the structure lacked all elements of symmetry and called: asymmetric.
Chirality (from the Greek word cheir, meaning “ hand Hand The hand constitutes the distal part of the upper limb and provides the fine, precise movements needed in activities of daily living. It consists of 5 metacarpal bones and 14 phalanges, as well as numerous muscles innervated by the median and ulnar nerves. Hand: Anatomy”) is the geometric property displayed by any object that is non-superimposable on its mirror image.
The main structural feature responsible for chirality is the presence of dissymmetry; as it has no plane of symmetry (as previously explained).
The best way to describe chirality is by looking at the right and left hands. They have the same number, size, and order of fingers, but their mirror images are not the same. The left hand Hand The hand constitutes the distal part of the upper limb and provides the fine, precise movements needed in activities of daily living. It consists of 5 metacarpal bones and 14 phalanges, as well as numerous muscles innervated by the median and ulnar nerves. Hand: Anatomy’s mirror image cannot be placed directly on top of the right hand Hand The hand constitutes the distal part of the upper limb and provides the fine, precise movements needed in activities of daily living. It consists of 5 metacarpal bones and 14 phalanges, as well as numerous muscles innervated by the median and ulnar nerves. Hand: Anatomy (hence, non-superimposable).
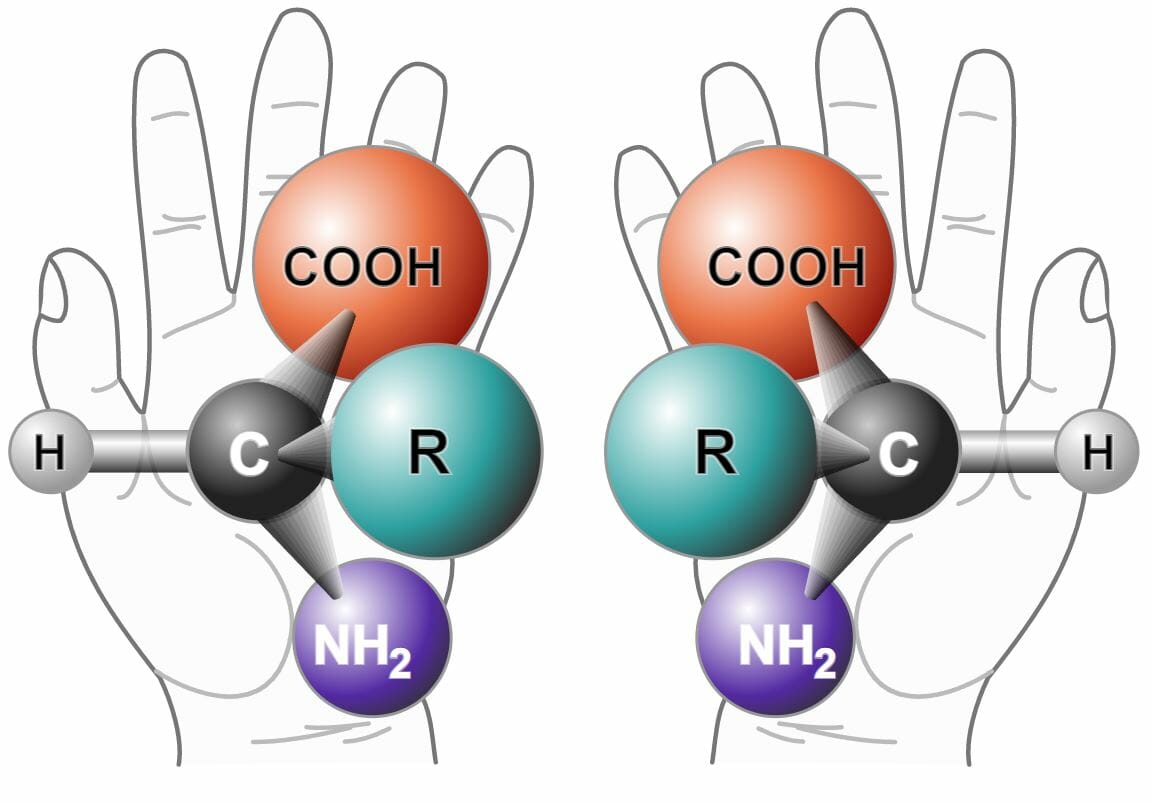
Two enantiomers of a generic amino acid that is chiral
Image: “Chirality with hands” by NASA. License: Public DomainChiral center (Stereogenic center, stereocenter) is a carbon atom that carries four different atoms, or a group of atoms.
Isomers are different compounds having the same molecular formula, but different structures. There are two types of Isomers:
There is a historical distinction between the two types of stereoisomers Stereoisomers The phenomenon whereby compounds whose molecules have the same number and kind of atoms and the same atomic arrangement, but differ in their spatial relationships. Basics of Carbohydrates: conformational isomers, which are interconvertible by rotations around single bonds, and configurational isomers, which do not interconvert at room temperature and, therefore, can be separated.
Configurational isomers encompass two entities:
1. Enantiomers:
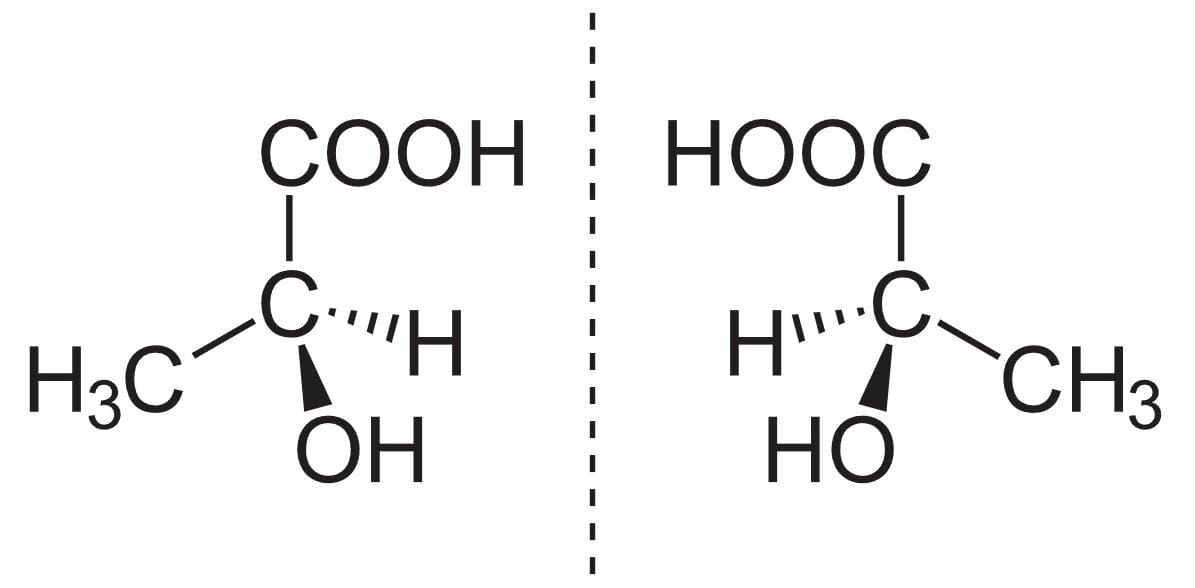
(S)-(+)-lactic acid (left) and (R)-(–)-lactic acid (right) are nonsuperposable mirror images of each other
Image: “Milchsäure Enantiomerenpaar” by NEUROtiker. License: Public DomainThey have the same physical and chemical properties except:
A) Direction of rotation Rotation Motion of an object in which either one or more points on a line are fixed. It is also the motion of a particle about a fixed point. X-rays of plane-polarized light:
B) They react differently with chiral compounds.
2. Diastereomers:
These are non-mirror image stereoisomers Stereoisomers The phenomenon whereby compounds whose molecules have the same number and kind of atoms and the same atomic arrangement, but differ in their spatial relationships. Basics of Carbohydrates. The physical properties and chemical reactions of diastereomers are different. A molecule that contains two asymmetric carbons (two stereocenters) is the most common diastereomer. Ephedrine and pseudoephedrine illustrate these compounds. Each diastereomer (ephedrine and pseudoephedrine) exists as a member of an enantiomeric pair: d- and l-ephedrine, and d- and l-pseudoephedrine:
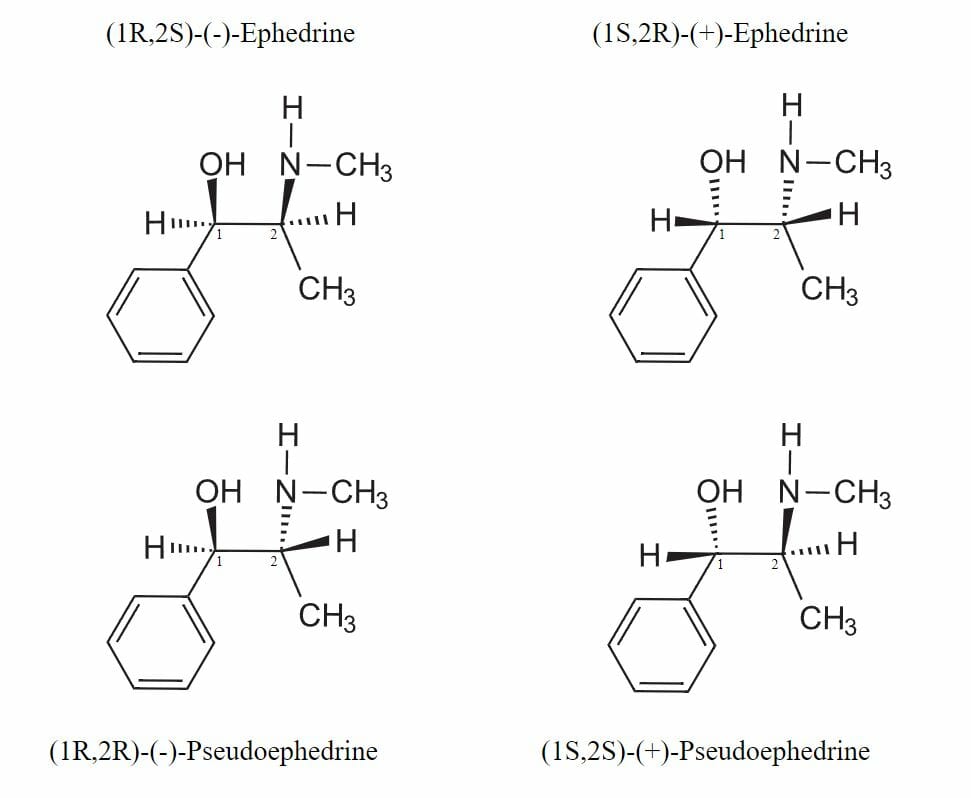
The four diastereoisomers of ephedrine
Image: “Ephedrine pseudoephedrine isomers” by Wickey-nl. License: Public DomainThus, diastereomeric molecules with two carbon centers are represented by four stereoisomers Stereoisomers The phenomenon whereby compounds whose molecules have the same number and kind of atoms and the same atomic arrangement, but differ in their spatial relationships. Basics of Carbohydrates.
One of the methods used in distinguishing one enantiomeric form from another is the evidence of rotation Rotation Motion of an object in which either one or more points on a line are fixed. It is also the motion of a particle about a fixed point. X-rays of plane-polarized light, which is shown as (+) / (-) or d / l. However, this method does not describe the spatial arrangement around the chiral center—the configuration.
A system of nomenclature has been established to represent the absolute configuration: the Cahn-Ingold-Prelog rules. These rules apply when the substituents around the chiral center are ordered (assigned priorities) from the largest to the smallest, according to their atomic number.
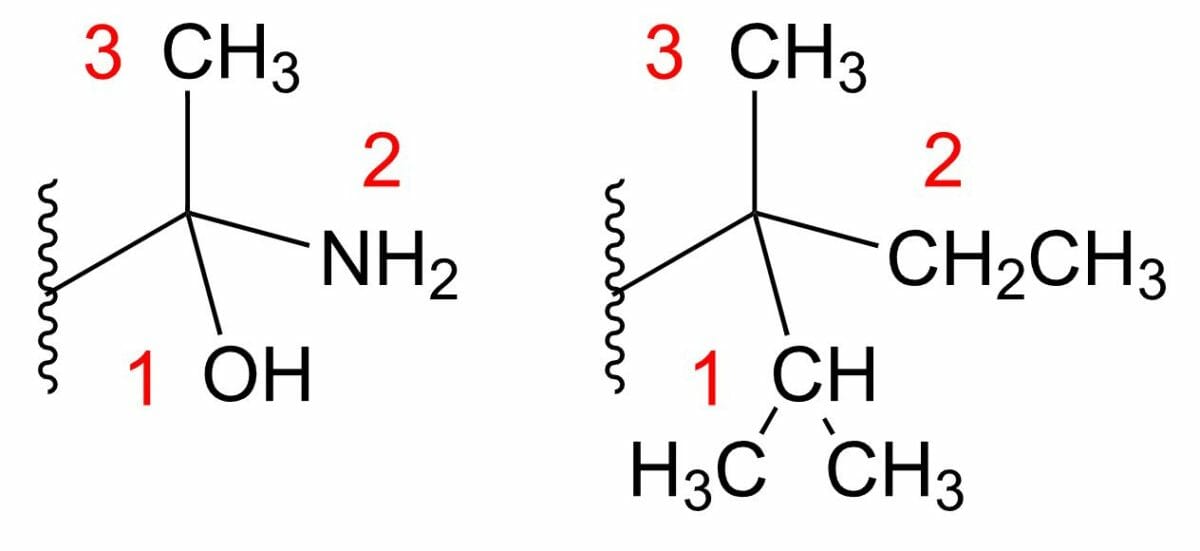
Two examples of stereocenters
The lowest substituent (number 4) is shown only by a wavy line and is assumed to be behind the rest of the molecule. Both centers shown are S isomers.
The lowest assigned priority group is placed towards the back. Then, the direction (clockwise or counter-clockwise) of a line connecting the remaining groups in descending order, from the highest priority to the lowest, is determined. Therefore:
Molecules with multiple chiral centers:
Nearly all of the biological environment consists of enantiomeric molecules— amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids, nucleosides Nucleosides Purine or pyrimidine bases attached to a ribose or deoxyribose. Nucleic Acids, carbohydrates Carbohydrates A class of organic compounds composed of carbon, hydrogen, and oxygen in a ratio of cn(H2O)n. The largest class of organic compounds, including starch; glycogen; cellulose; polysaccharides; and simple monosaccharides. Basics of Carbohydrates, and phospholipids Phospholipids Lipids containing one or more phosphate groups, particularly those derived from either glycerol (phosphoglycerides) or sphingosine (sphingolipids). They are polar lipids that are of great importance for the structure and function of cell membranes and are the most abundant of membrane lipids, although not stored in large amounts in the system. Lipid Metabolism are all chiral molecules.
It has been shown that one enantiomer of a molecule can be much more active, pharmacologically, than another enantiomer.
In 1933, Easson and Stedman proposed that only three groups (b, c, d) out of the four (a,b,c,d) arranged on the central carbon of an enantiomer can be involved in the process of reaction. The receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors has three groups (b’, c’, d‘). The maximum physiological effect of a drug occurred when the groups b, c, and d in the drug coincide, respectively, with b‘, c‘ and d‘ in the receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors.
The action of the enantiomers of the epinephrine Epinephrine The active sympathomimetic hormone from the adrenal medulla. It stimulates both the alpha- and beta- adrenergic systems, causes systemic vasoconstriction and gastrointestinal relaxation, stimulates the heart, and dilates bronchi and cerebral vessels. Sympathomimetic Drugs supported these results. It was determined that (-)-epinephrine is 12-15 times as active as (+)-epinephrine.
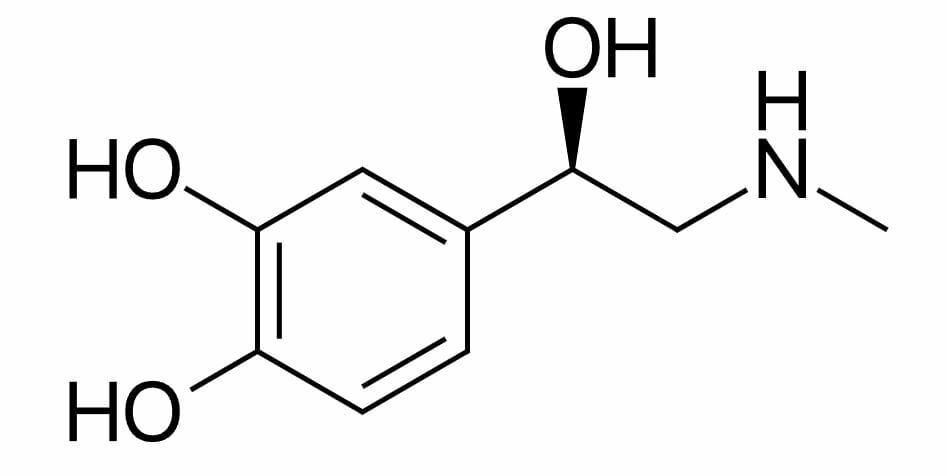
Skeletal formula of epinephrine (adrenaline)
Image: “Epinephrine” by Roland Mattern. License: Public DomainThalidomide Thalidomide A piperidinyl isoindole originally introduced as a non-barbiturate hypnotic, but withdrawn from the market due to teratogenic effects. It has been reintroduced and used for a number of immunological and inflammatory disorders. Thalidomide displays immunosuppressive and anti-angiogenic activity. It inhibits release of tumor necrosis factor-alpha from monocytes, and modulates other cytokine action. Immunosuppressants was first introduced in Germany in 1957. It was used to alleviate morning sickness in pregnant women. However, it was soon discovered to cause birth malformations such as phocomelia, Amelia Amelia The absence of one or both limbs, which occurs due to suspension in development during the 4th week. Development of the Limbs and, in extreme situations, death. Re-examination of its formulaic property revealed that the two enantiomers of Thalidomide Thalidomide A piperidinyl isoindole originally introduced as a non-barbiturate hypnotic, but withdrawn from the market due to teratogenic effects. It has been reintroduced and used for a number of immunological and inflammatory disorders. Thalidomide displays immunosuppressive and anti-angiogenic activity. It inhibits release of tumor necrosis factor-alpha from monocytes, and modulates other cytokine action. Immunosuppressants exert different effects:
This is because each one fits a different active site Active site Area of an enzyme that binds to specific substrate molecules in order to facilitate a reaction. Basics of Enzymes of a specific enzyme, producing a different biological effect.
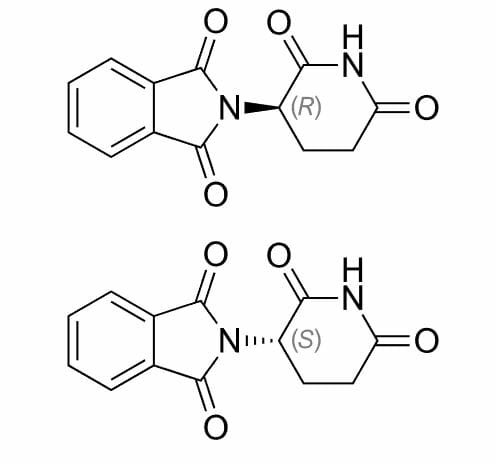
Enantiomers of thalidomide
Image: “Thalidomide enantiomers” by Vaccinationist. License: Public DomainEnzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body’s constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes are chiral molecules and can be distinguished between the two enantiomers of a chiral substrate Substrate A substance upon which the enzyme acts. Basics of Enzymes. This is similar to the right glove fitting exactly onto the right hand Hand The hand constitutes the distal part of the upper limb and provides the fine, precise movements needed in activities of daily living. It consists of 5 metacarpal bones and 14 phalanges, as well as numerous muscles innervated by the median and ulnar nerves. Hand: Anatomy, but poorly fitting the left hand Hand The hand constitutes the distal part of the upper limb and provides the fine, precise movements needed in activities of daily living. It consists of 5 metacarpal bones and 14 phalanges, as well as numerous muscles innervated by the median and ulnar nerves. Hand: Anatomy.
A final example of chirality is the two enantiomers of the chemical compound carvone, in which:
Therefore, in the human body (chiral environment), two different enantiomer types can produce entirely different biological effects.