Carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance are a group of compounds containing the functional group –COOH and represent a large class of organic compounds with varying applications from numerous natural products to synthetic drugs and hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types. This article provides a review of the simple nomenclature and reactivity of carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance, as well as their participation in nucleophilic substitution reactions and the formation of carboxylic acid derivatives.
Last updated: Mar 7, 2022
General formula:
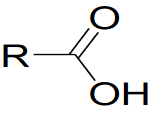 Nomenclature: In the naming of carboxylic
acids
Acids
Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water.
Acid-Base Balance, the –e at the end of the corresponding alkane is replaced with –oic acid. Carboxylic
acids
Acids
Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water.
Acid-Base Balance, RCOOH, are one of the most commonly encountered organic compounds. The reactivities of carboxylic
acids
Acids
Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water.
Acid-Base Balance are centered around the –COOH functional group, specifically due to the inherent polarity of the carbonyl (C=O) group contained within it. The most electron-rich site for the compound is the carbonyl oxygen, while the most electron-poor site is the hydrogen atom attached to the OH group, resulting in the relative polarity of the compound.The –COOH functional group also affects the physical properties of the compound. Carboxylic
acids
Acids
Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water.
Acid-Base Balance have relatively higher boiling and melting points compared to hydrocarbons and other oxygen-containing organic compounds of similar size and shape.The main factor contributing to the differences in the physical properties of carboxylic
acids
Acids
Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water.
Acid-Base Balance is the possibility of
hydrogen bonding
Hydrogen bonding
A low-energy attractive force between hydrogen and another element. It plays a major role in determining the properties of water, proteins, and other compounds.
DNA Types and Structure through two oxygen atoms present within its structure. The 2 oxygen atoms are tightly held by hydrogen bonds, making the compound harder to convert from one state to another.
Nomenclature: In the naming of carboxylic
acids
Acids
Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water.
Acid-Base Balance, the –e at the end of the corresponding alkane is replaced with –oic acid. Carboxylic
acids
Acids
Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water.
Acid-Base Balance, RCOOH, are one of the most commonly encountered organic compounds. The reactivities of carboxylic
acids
Acids
Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water.
Acid-Base Balance are centered around the –COOH functional group, specifically due to the inherent polarity of the carbonyl (C=O) group contained within it. The most electron-rich site for the compound is the carbonyl oxygen, while the most electron-poor site is the hydrogen atom attached to the OH group, resulting in the relative polarity of the compound.The –COOH functional group also affects the physical properties of the compound. Carboxylic
acids
Acids
Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water.
Acid-Base Balance have relatively higher boiling and melting points compared to hydrocarbons and other oxygen-containing organic compounds of similar size and shape.The main factor contributing to the differences in the physical properties of carboxylic
acids
Acids
Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water.
Acid-Base Balance is the possibility of
hydrogen bonding
Hydrogen bonding
A low-energy attractive force between hydrogen and another element. It plays a major role in determining the properties of water, proteins, and other compounds.
DNA Types and Structure through two oxygen atoms present within its structure. The 2 oxygen atoms are tightly held by hydrogen bonds, making the compound harder to convert from one state to another.
Carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance are considered weak acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance as they can donate a proton (H+), to a small extent. When a proton is eliminated from the –OH group of the compound, the electrons in the remaining –COO– group exhibit resonance, causing a slight stabilization of the resulting anion.
Common replacements of the –OH group:
Below are some examples of these carboxylic acid derivatives.
The simple way of naming carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance is by counting the number of carbons in the longest continuous chain that includes the carboxyl carbon. The –e in the alkane or alkene name corresponding to the identified number of carbons is then replaced with –oic acid. The table below shows some of the common carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance with their systematic names.
In most of the examples above, the only prominent functional group is the carboxylic acid group.
However, it is also possible to have a carboxylic acid with one or more other functional groups present in the chain. For example, hydroxyethanoic acid would mean Mean Mean is the sum of all measurements in a data set divided by the number of measurements in that data set. Measures of Central Tendency and Dispersion an ethanoic acid with another hydroxy group.
In naming organic compounds, the functional group given the highest priority is the carboxylic acid group. The carbon where the other functional groups are attached is assigned numbers, with the carbonyl carbon of the carboxylic acid group assigned as the carbon 1. In writing their names, the substituents are arranged in alphabetical order, regardless of their carbon number. Below are some of the examples of mono and di-substituted carboxylic acid.
In cases where the compound has 2 carboxylic acid groups on both sides of the longest carbon chain, the suffix –dioic acid is added to the alkane name for the longest carbon chain. Below is the structure of butanedioic acid:
Because of the inherent polarity of the carbonyl C=O bond, carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance are prone to attack via nucleophilic substitution reaction. Just like in aldehydes Aldehydes Organic compounds containing a carbonyl group in the form -cho. Basics of Carbohydrates, the nucleophile attacks the carbonyl carbon, forming a tetrahedral intermediate and, in the process, producing different possible carboxylic acid derivatives. In a nucleophilic acyl substitution reaction, elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy of one of the 2 substituents attached to the carbonyl carbon takes place as the nucleophile attaches itself to that carbon atom.
Unlike in aldehydes Aldehydes Organic compounds containing a carbonyl group in the form -cho. Basics of Carbohydrates and ketones Ketones Organic compounds containing a carbonyl group =C=O bonded to two hydrocarbon groups. Basics of Carbohydrates where an –H or other alkyl substituent is attached to the carbonyl carbon, substituents in a carboxylic acid (or its derivatives) are good leaving groups. Since the –H and alkyl groups in aldehyde or ketones Ketones Organic compounds containing a carbonyl group =C=O bonded to two hydrocarbon groups. Basics of Carbohydrates are not good leaving groups, they undergo nucleophilic addition, whereas carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance undergo nucleophilic substitution. The general mechanism for this type of reaction is shown in the figure:
The production of carboxylic acid derivatives is a two-step reaction, each of which is important in predicting the reactivity of the compound. Furthermore, electronic and steric factors are also important to consider when comparing reactivities of carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance and their derivatives.
Steric factors are an important consideration for assessing reactivity because the presence of bulky groups significantly affects the rate of the first step in derivative production; the bulkier the alkyl group attached to the carbonyl carbon, the harder it is for the nucleophile to attack the carbonyl carbon.
The polarization of acyl compounds is also important in predicting the reactivities. More strongly polarized acyl compounds react more readily than less polar ones. This means, for example, acyl chlorides will react faster in nucleophilic substitution compared to the less polarized amides.
When carboxylic acid derivatives react with water, all reactions lead to a production of carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance. This is because groups like –NH2 and –Cl, for example, are better-leaving groups compared to –OH. Therefore, when the water molecule attaches itself to the carbonyl carbon, the leaving groups are readily eliminated together with an H+ from the water. Below is the mechanism for the hydrolysis Hydrolysis The process of cleaving a chemical compound by the addition of a molecule of water. Proteins and Peptides of acyl chloride Chloride Inorganic compounds derived from hydrochloric acid that contain the Cl- ion. Electrolytes. The same mechanism is followed by other carboxylic acid derivatives.
An aminolysis reaction is possible for esters using ammonia Ammonia A colorless alkaline gas. It is formed in the body during decomposition of organic materials during a large number of metabolically important reactions. Note that the aqueous form of ammonia is referred to as ammonium hydroxide. Acid-Base Balance or amines as nucleophiles. However, this reaction is not commonly employed, as the use of acyl halides lead to faster reactions.