Chemical reactions occur as chemical compounds form and break apart. In a closed system, equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy results after a certain period of time. From this point forward, no changes in the concentration of the products and reagents can be observed anymore. In the following article, you learn which requirements are necessary for this state, what exactly characterizes this state, which meaning it has, and how one influences and uses it for certain purposes.
Last updated: Mar 7, 2022
Chemical equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy is the state of a chemical system at which a constant concentration of products and reagents is present. Reactions, which take place in homogeneous Homogeneous Imaging of the Spleen solutions, seem to have come to rest because no changes in concentrations of the participating substances can be determined. Substance turnover occurs only on the particle level, which is why chemical equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy is also referred to as dynamic equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy.
For each reaction, the position of equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy, under certain surrounding conditions, is determined by a natural constant.
This form of reaction is also known as a reversible reaction, as it occurs in both directions and simultaneously. This condition results in the reaction equation for the reaction type of the equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy reaction to contain a double arrow. However, reversible reactions can take place only if none of the reaction partners leave the system.
In order to explain the equilibration of a reaction, be conscious of the meaning of the term reaction velocity. Many reactions can go backward, as well as forward. Reaction velocity is the change in substance concentration during a certain period of time. If forward and backward reactions occur simultaneously, which is typical for an equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy reaction, the following applies:
A + B ⇔ C + D
Vforward = k1 • cA • cB
Vbackward = k2 • cC • cD
(k= proportionality factor, c = concentration)
Thus, the following applies in chemical equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy:
Vforward = vbackward, and k1 • cA • cB = k2 • cC • cD
If a reaction takes place incompletely in a closed system and is also reversible ( equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy reaction), the reaction initially has a high reaction velocity, as the concentrations of the original substances are high. The reaction velocity of the forward reaction gradually decreases because the substance concentrations of the reagents constantly decrease, while the backward reaction gains velocity because the substance concentrations of the products increase in the course of the reaction.
This process swings back and forth until a state is reached at which the same amount of products and reagents is formed. In this state, the velocities of the forward and backward reactions are equal. This is why the reaction seems to have halted. Macroscopically, no changes can be observed as the chemical transitions occur only on the particle level.
The position of the chemical equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy is specific for each reaction and corresponds to a natural constant, which means that it cannot be changed. However, the time for equilibration can be shortened with the help of catalyzers.
Equilibration time is also specific for each reaction, but only at constant temperature conditions. Shortening of the time can be explained by the ability of catalyzers to cause more ‘effective collisions’ in their active state so that the chemical reaction is accelerated.
The law of mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast action, or LMA, offers the mathematical instrument to quantitatively describe the position of chemical equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy.
If more than 50% of the original substances react to products, the equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy is rather on the right side of the overall picture, referred to as equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy located on the right side.
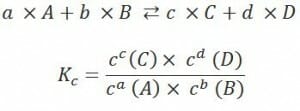
k =
equilibrium
Equilibrium
Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response.
Cancer Immunotherapy or
mass
Mass
Three-dimensional lesion that occupies a space within the breast
Imaging of the Breast action constant
c = substance concentration
A, B, C, D = reaction partners or their substance concentrations
a, b, c, d = stoichiometric numbers; can be taken from the reaction equation
One main statement of the LMA is that the relation k of the multiplied product Product A molecule created by the enzymatic reaction. Basics of Enzymes concentrations to the multiplied reagent concentrations is constant for certain reactions under determined conditions. Thus, the quotient K of this equation is also referred to as equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy or mass Mass Three-dimensional lesion that occupies a space within the breast Imaging of the Breast action constant.
However, one must consider that the LMA can be applied only to dilute solutions. In more concentrated solutions, there are deviations between the particles due to interactions. For example, OH ions cannot move in strong bases Bases Usually a hydroxide of lithium, sodium, potassium, rubidium or cesium, but also the carbonates of these metals, ammonia, and the amines. Acid-Base Balance, as there is not enough solvent present for the ions. This makes one conclude that the position of the equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy fluctuates, depending on the concentration. Yet, the same concentrations develop in the equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy according to the LMA, which is why one always considers diluted solutions as ‘reference’.
Furthermore, the location of equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy depends on temperature and, possibly, on pressure relations. The section below discussing disturbances of chemical equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy will provide further explanation.
The calculated equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy constant Kc has great importance for further calculations. On the bases Bases Usually a hydroxide of lithium, sodium, potassium, rubidium or cesium, but also the carbonates of these metals, ammonia, and the amines. Acid-Base Balance of the value of Kc, one can calculate the transformed amount of reagents or the exploit of reaction products. Kc can be determined from experimental values.
If a chemical equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy is disturbed, an acceleration of the reaction occurs, which then eliminates or reverses the disturbance. This rule is also known as the principle of least constraint, or Le Châtelier’s principle. The “constraint” refers to the disturbance of the equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy, which leads to the reaction having to be compensated by acceleration.
“If one applies a constraint to a system in equilibrium, the system shifts in the direction of a new equilibrium level so that the effect of the constraint becomes minimal. That is the smallest.”
A disturbance can be triggered by different factors. As mentioned previously, the position of the equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy can be changed by the deviation of temperature and pressure conditions. Also, participating in substance amounts also have an influence.
The following section examined other factors to illustrate the way changes can be triggered by different factors.
Energy input (e.g., via heating) results in a reinforced ‘uphill’ reaction. An increase in the formation of reagents occurs, which actually forms products, which store their energy there, under ‘normal’ conditions.
This means that an increase in temperature promotes an endothermic reaction and that the value of the equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy constant decreases. Analogously, the opposite happens in the event of a decrease in energy or temperature: The location of the equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy shifts in the direction of the products and the exothermic reaction is promoted.
For the sake of ‘rescue’, the following reactions occur with the adding or removal of reaction partners: [reaction: A + B → C + D].
An increase in the concentration of a substance promotes its consumption and a decrease in concentration promotes its reproduction.
Via adding of acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance, bases Bases Usually a hydroxide of lithium, sodium, potassium, rubidium or cesium, but also the carbonates of these metals, ammonia, and the amines. Acid-Base Balance, or precipitants, the concentration of reaction partners can, however, also be disturbed. In such a case, 2 coupled equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy reactions often occur parallel.
If the participating substances in an equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy reaction in a closed system are gasses, a change in pressure results in a change in the location of the chemical equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy. If the reaction partners have another aggregate phase than gaseous, the equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy is not affected or changed. The background of this phenomenon is that changes in volume at reactions with non-gaseous substances are so small that the dependency of the location of the equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy on the pressure can be neglected.
If an increase in pressure occurs during a reaction that takes place under a decrease in volume, the chemical equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy shifts to the side of the products. An increase in pressure during a reaction that takes place under an increase in volume leads to the location of the equilibrium Equilibrium Occurs when tumor cells survive the initial elimination attempt These cells are not able to progress, being maintained in a state of dormancy by the adaptive immune system. In this phase, tumor immunogenicity is edited, where T cells keep selectively attacking highly immunogenic tumor cells.This attack leaves other cells with less immunogenicity to potentially develop resistance to the immune response. Cancer Immunotherapy to be shifted to the reagents.
A decrease in pressure promotes the reaction, which occurs under an increase in volume.