Nursing Knowledge
Acid-base disorders involve imbalances in the body’s pH levels, often categorized as acidosis (low pH) or alkalosis (high pH). These disorders can be respiratory or metabolic in origin, and they are commonly identified through arterial blood gas (ABG) analysis.
Uncompensated:
Opposite system is not responding, pH remains imbalanced.
Partial compensation:
Opposite system is working to correct the imbalance, pH not yet normalized.
Full compensation:
Homeostasis achieved, all lab values return to normal.
Respiratory alkalosis is a decrease in carbon dioxide levels due to increased excretion by the lungs. Excess carbon dioxide leads to pH imbalance.
The most common cause of respiratory alkalosis is hyperventilation.
| Normal values | Respiratory alkalosis | |
| pH | 7.35–7.45 | Increased |
| CO2 | 35–45 mm Hg | Decreased |
| HCO3- | 22–26 mmol/L | Normal or decreased |
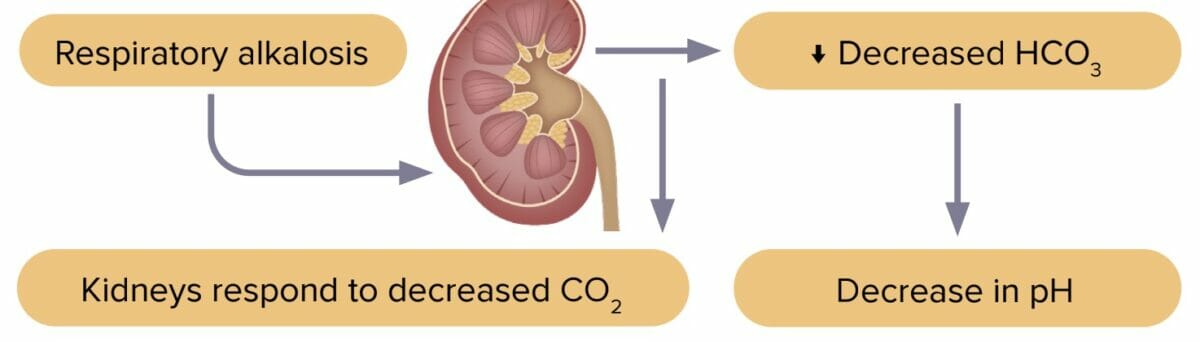
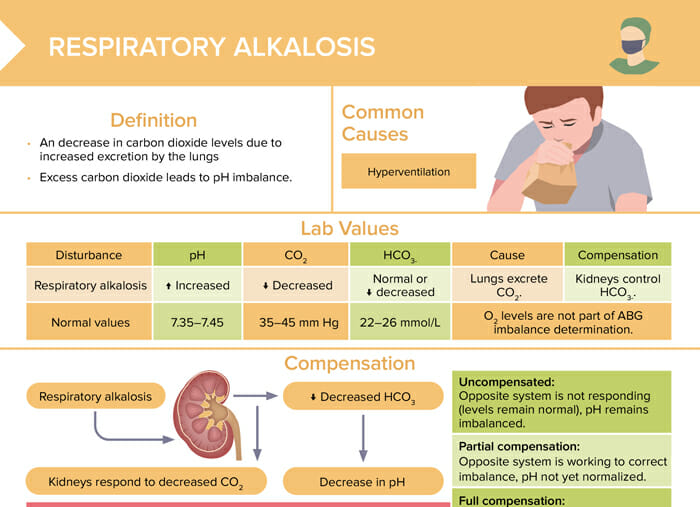
Reviews definition, causes and lab values and treatment of respiratory alkalosis
Respiratory acidosis is an accumulation of carbon dioxide in the body due to decreased excretion by the lungs. Decreased carbon dioxide levels lead to pH imbalance.
The most common causes of respiratory acidosis are:
| Normal values | Respiratory acidosis | |
| pH | 7.35–7.45 | Decreased |
| CO2 | 35–45 mm Hg | Increased |
| HCO3- | 22–26 mmol/L | Normal or decreased |
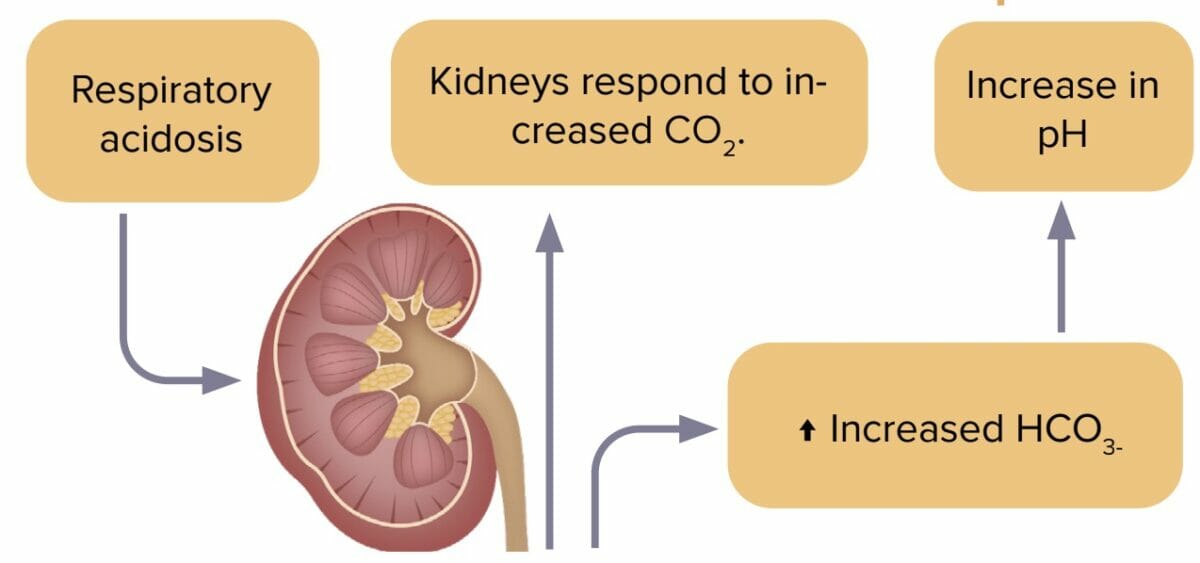
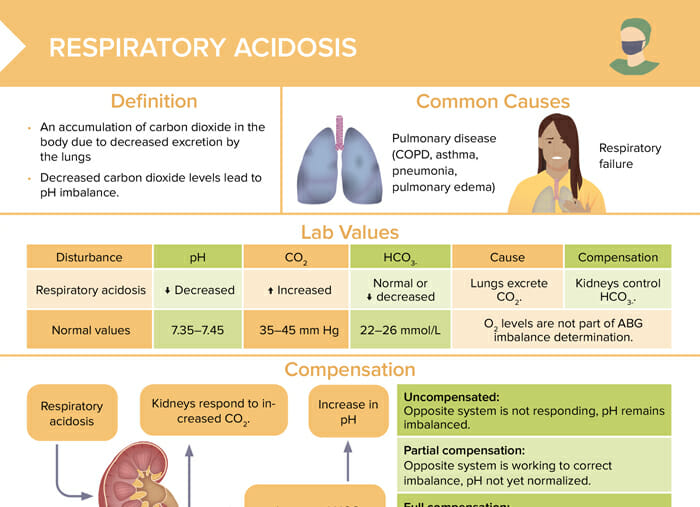
Reviews definition, causes and lab values and treatment of respiratory acidosis
Metabolic alkalosis is an accumulation of bicarbonate in the body caused by loss of stomach acid. Excess bicarbonate leads to pH imbalance. Metabolic alkalosis is usually accompanied by hypokalemia.
The most common cause of metabolic alkalosis is a loss of stomach acid, caused by excessive NG suction or vomiting.
| Normal values | Metabolic alkalosis | |
| pH | 7.35–7.45 | Increased |
| CO2 | 35–45 mm Hg | Normal or increased |
| HCO3- | 22–26 mmol/L | Increased |
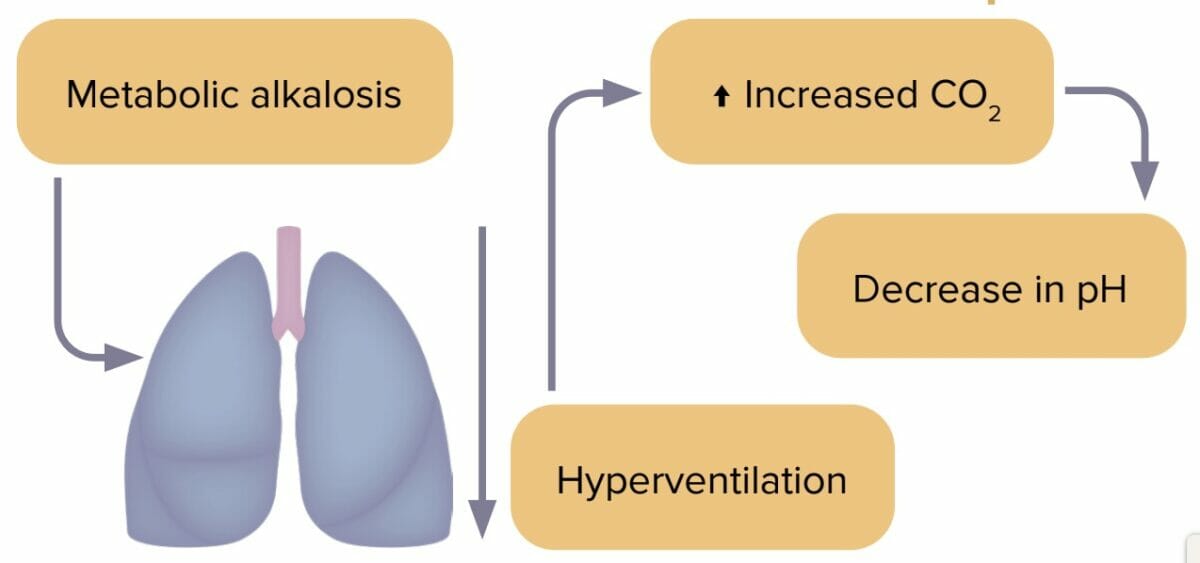
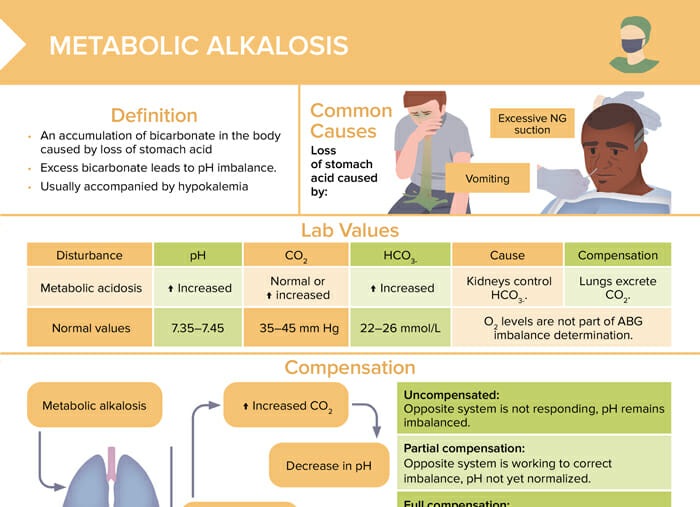
Reviews definition, causes and lab values and treatment of metabolic alkalosis
Metabolic acidosis is an accumulation of acid in the body caused by either increased acid generation, loss of bicarbonate, or diminished renal acid excretion. Excess acid and decreased bicarbonate lead to pH imbalance.
The most common causes of metabolic acidosis are:
| Normal values | Metabolic acidosis | |
| pH | 7.35–7.45 | Decreased |
| CO2 | 35–45 mm Hg | Normal or decreased |
| HCO3- | 22–26 mmol/L | Decreased |
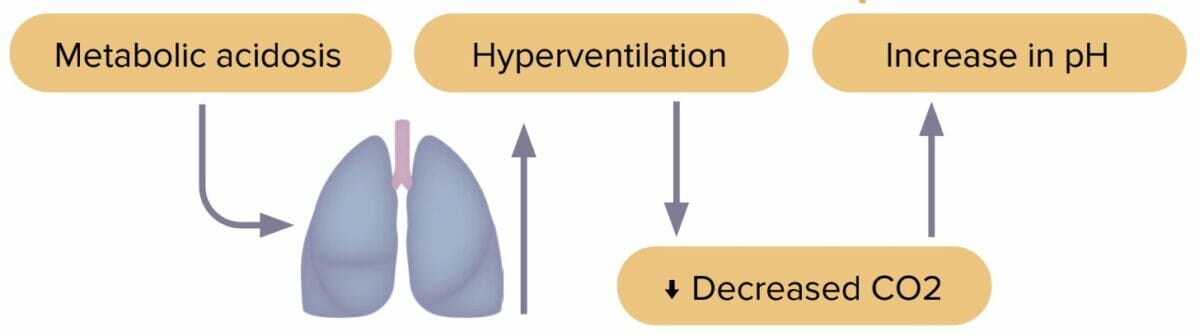
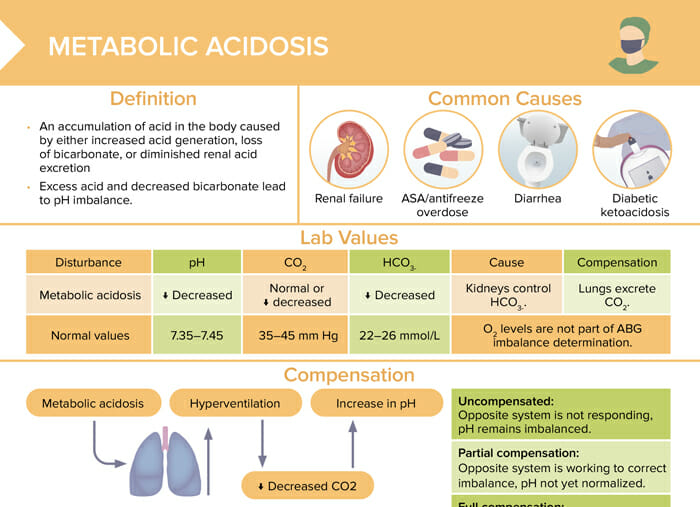
Reviews definition, causes and lab values and treatment of metabolic acidosis
| Disturbance | pH | CO2 | HCO3- |
| ??? | 7.48 | 27 | 19 |
Answer: Respiratory alkalosis partially compensated – HCO3 (metabolic component) is low (19 mEq/L), which is an attempt to lower the pH and counteract the respiratory alkalosis; pH is still outside the normal range (above 7.45), but the HCO3 is actively trying to correct the imbalance.
| Disturbance | pH | CO2 | HCO3- |
| ??? | 7.30 | 55 | 25 |
Answer: Respiratory acidosis uncompensated – pH is still outside the normal range; caused by a primary problem with the respiratory system that leads to the retention of CO₂, making the blood acidic; kidneys (metabolic system) have not yet responded significantly to correct the imbalance.
| Disturbance | pH | CO2 | HCO3- |
| ??? | 7.47 | 47 | 30 |
Answer: Metabolic alkalosis fully compensated – Abnormal PaCO₂ (high) indicates that the respiratory system is working to compensate for the metabolic alkalosis by retaining CO₂, which increases the acidity of the blood, bringing the pH back toward the normal range.
| Disturbance | pH | CO2 | HCO3- |
| ??? | 7.25 | 32 | 18 |
Answer: Metabolic acidosis partially compensated – CO2 level (32 mmHg) is low, which is an attempt by the respiratory system to compensate for the metabolic acidosis. The body “blows off” CO2 (an acid) to raise the pH closer to the normal range.
Mixed acid-base disorders occur when a patient has more than one primary acid-base imbalance simultaneously. For example, a patient could have both respiratory acidosis and metabolic alkalosis. These disorders are often more complex to diagnose and manage, requiring careful interpretation of arterial blood gas (ABG) results alongside clinical assessment.
| Normal | Respiratory acidosis | Respiratory alkalosis | Metabolic acidosis | Metabolic alkalosis | |
| pH | 7.35–7.45 | Decreased | Increased | Decreased | Increased |
| CO2 | 35–45 mm Hg | Increased | Decreased | Normal or decreased | Normal or increased |
| HCO3- | 22–26 mmol/L | Normal or decreased | Normal or decreased | Decreased | Increased |
Free Download
Master the topic with a unique study combination of a concise summary paired with video lectures.
Your free account gives you access to:
or