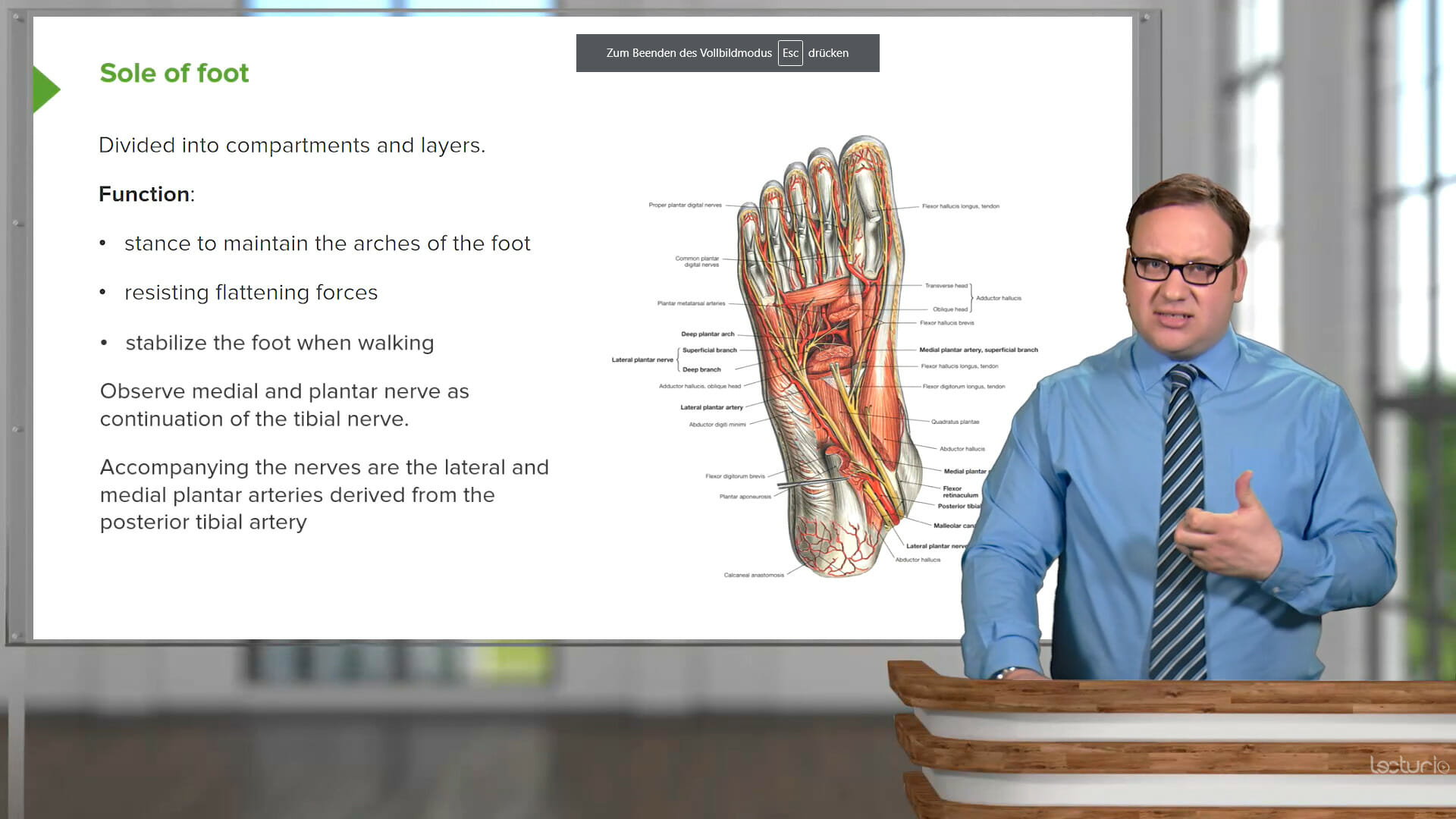
Study with the MCAT chemistry and physics review course
geared towards optimal preparation for the MCAT section “Chemical and Physical Foundations of Biological Systems”
Understanding chemical and physical foundations is a prerequisite for succeeding in medical school and tested intensely on the MCAT. However, the integration of complex physics and chemistry concepts into biological contexts can be particularly challenging for many students.
This course delves into core concepts of physics and chemistry, from the fluid mechanics of blood and the bioelectricity of membrane potentials to the exothermic reactions involved in breaking down ATP. Your educators are an experienced team of experts in their respective fields who go beyond explaining the topics, encouraging you to think critically and apply the concepts to real problems and questions.
The combination of Video Lessons with interactive quiz questions, downloadable study materials, and a MCAT-style Qbank makes it easy to understand and retain the topics. By the end of this course, you will have a thorough grasp of the chemical and physical principles essential for the MCAT, preparing you not only for the exam but also for the rigorous demands of medical school.