Raise Your MCAT® Score and Increase Your Chances of Acceptance
Experience unmatched MCAT preparation with expert-crafted review courses, practice tests, and admission coaching.
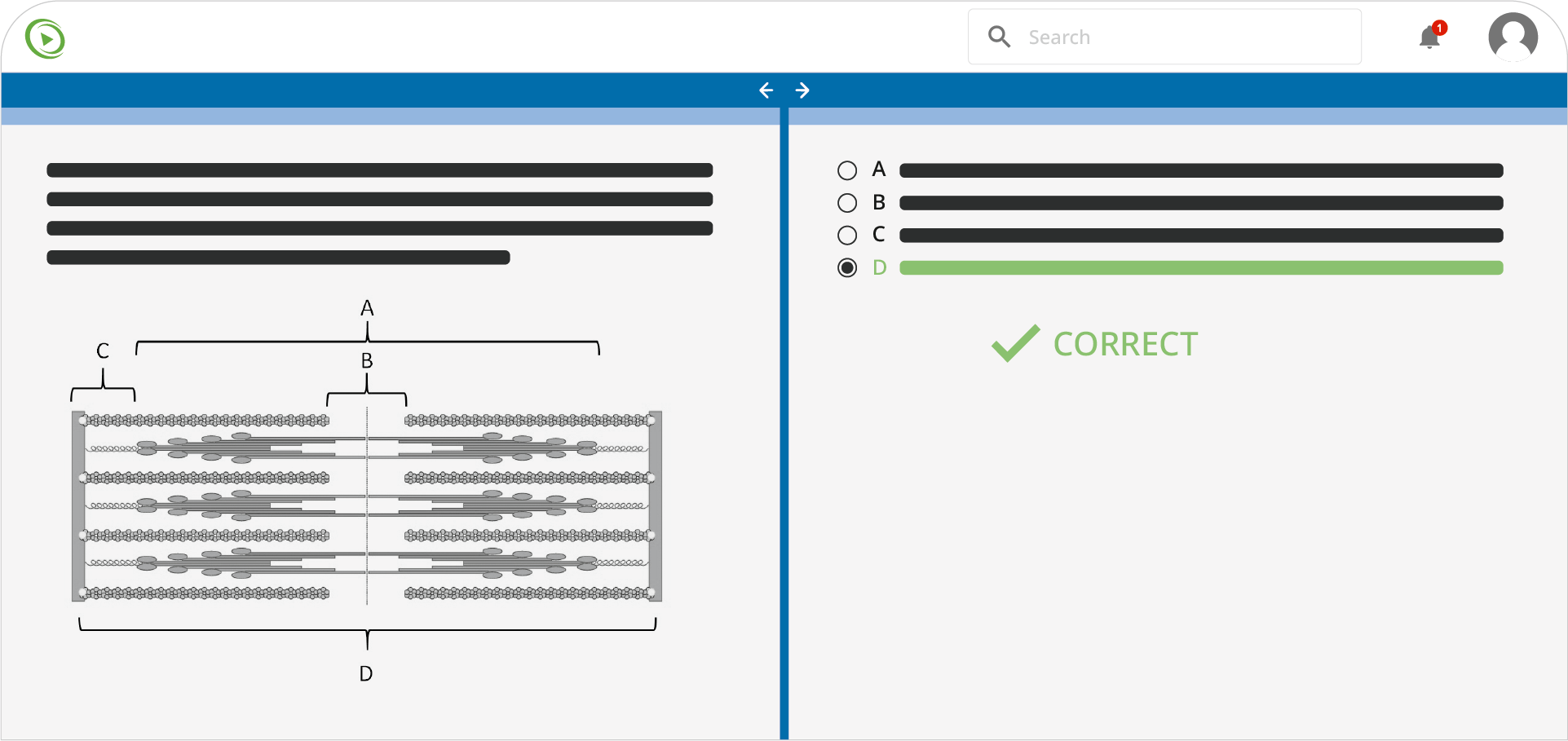
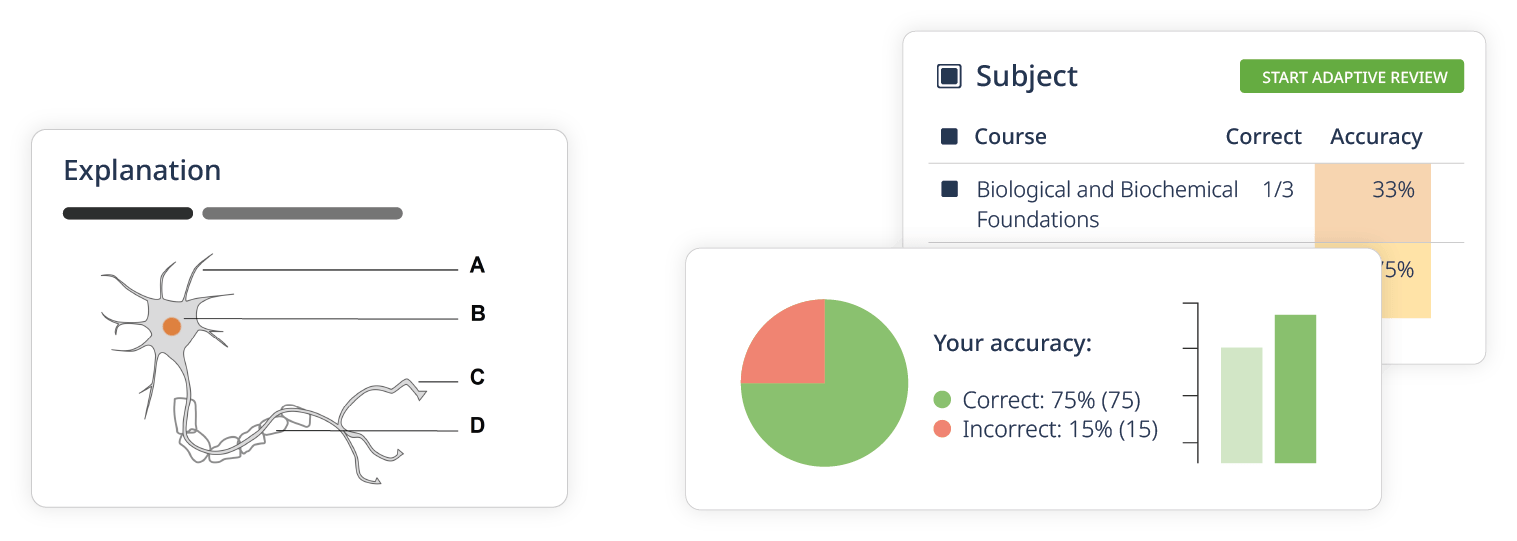

Learning Paths
Practice Your Way to Success
Guided MCAT preparation with an AI-augmented Qbank and expert-crafted learning paths
- 4,100+ Qbank questions
- In-depth text rationales
- AI Tutor assistance
- Performance tracking with adaptive review
Lecturio’s expansive Qbank is a crucial tool for mastering the MCAT. Practice with confidence and solidify your understanding of key concepts.



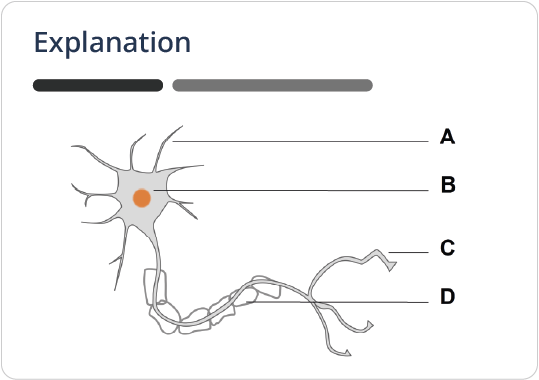




Available Learning Paths
- Full MCAT Practice Exams
- Free MCAT Sample Test
Navigate your MCAT prep with Lecturio’s Learning Paths, curated by top MCAT coaches. These guides and practice exams provide clear instructions on content and questions for a strategically planned journey towards your exam success.
Unlock the Secrets to Medical School Acceptance: Expert Tips and Strategies
Discover how to increase your chances of getting into the medical school of your dreams with Lecturio's insider knowledge and guidance

Achieve Your Best MCAT Score with Lecturio
Our comprehensive high-yield videos and concept pages are designed to provide you with the complete resources you need to succeed on the MCAT
Video Library Highlights
- 1,000+ comprehensive video lessons
- Integrated recall questions
- High-quality and downloadable slides
- Detailed illustrations
- Summaries to support your learning
Lecturio is dedicated to simplifying complex MCAT topics through engaging video lessons taught by expert educators, providing in-depth coverage for a thorough understanding.








Concept Page Library
- 300+ in-depth Concept Pages
- Linked video lessons and Qbank tests
- High-yield and up-to-date
- Detailed images, charts, and tables
Reinforce your knowledge on challenging MCAT topics using our comprehensive Concept Pages, created and peer-reviewed by US-trained physicians.

Lecturio works
Lecturio’s award-winning educators, learning experts, and physicians work together to integrate evidence-based learning tools and strategies into your MCAT preparation
20 point increase
In NBME CBSSA scores using supplemental study routines from Lecturio
37% increase in correct answers
When answering first attempt Qbank questions

37% increase
in correct answers
When answering first attempt Qbank questions
Increased their exam confidence
in their preparation for MCAT
Preferred the increased flexibility and convenience Lecturio offers
in exam preparation after using Lecturio
Would recommend Lecturio to a friend
An overwhelming number of students find Lecturio to be a smart and reliable resource
Have a holly, jolly study session
Get
50%
off all plans
MCAT Basic
Full library access
Videos + Concept Pages
without Qbank and smart exam prep features
Best for MCAT prep
MCAT Premium
Full library access
Videos + Concept Pages
Powerful Qbank
with AI-tutor + performance tracking
Structured study plans
Expert-built exam & board prep
Practice exams
Manually curated learning paths
AI Assistant
Get answers you can trust
Personal Tutoring
starts at
per month
A personal study tutor helps you achieve your goals in regular 1-on-1 tutoring sessions.
MCAT Basic
Pay after 7 days, cancel anytime
Full library access
Videos + Concept Pages
without Qbank and smart exam prep features
Best for MCAT prep
MCAT Premium
Pay after 7 days, cancel anytime
Full library access
Videos + Concept Pages
Powerful Qbank
with AI-tutor + performance tracking
Structured study plans
Expert-built exam & board prep
Practice exams
Manually curated learning paths
AI Assistant
Get answers you can trust
Personal Tutoring
starts at
per month
A personal study tutor helps you achieve your goals in regular 1-on-1 tutoring sessions.
MCAT Basic
Full library access
Videos + Concept Pages
without Qbank and smart exam prep features
Best for MCAT prep
MCAT Premium
Full library access
Videos + Concept Pages
Powerful Qbank
with AI-tutor + performance tracking
Structured study plans
Expert-built exam & board prep
Practice exams
Manually curated learning paths
AI Assistant
Get answers you can trust
Personal Tutoring
starts at
per month
A personal study tutor helps you achieve your goals in regular 1-on-1 tutoring sessions.
MCAT Basic
Pay after 7 days, cancel anytime
Full library access
Videos + Concept Pages
without Qbank and smart exam prep features
Best for MCAT prep
MCAT Premium
Pay after 7 days, cancel anytime
Full library access
Videos + Concept Pages
Powerful Qbank
with AI-tutor + performance tracking
Structured study plans
Expert-built exam & board prep
Practice exams
Manually curated learning paths
AI Assistant
Get answers you can trust
Personal Tutoring
starts at
per month
A personal study tutor helps you achieve your goals in regular 1-on-1 tutoring sessions.
MCAT Basic
Full library access
Videos + Concept Pages
without Qbank and smart exam prep features
Best for MCAT prep
MCAT Premium
Full library access
Videos + Concept Pages
Powerful Qbank
with AI-tutor + performance tracking
Structured study plans
Expert-built exam & board prep
Practice exams
Manually curated learning paths
AI Assistant
Get answers you can trust
Personal Tutoring
starts at
per month
A personal study tutor helps you achieve your goals in regular 1-on-1 tutoring sessions.
MCAT Basic
Pay after 7 days, cancel anytime
Full library access
Videos + Concept Pages
without Qbank and smart exam prep features
Best for MCAT prep
MCAT Premium
Pay after 7 days, cancel anytime
Full library access
Videos + Concept Pages
Powerful Qbank
with AI-tutor + performance tracking
Structured study plans
Expert-built exam & board prep
Practice exams
Manually curated learning paths
AI Assistant
Get answers you can trust
Personal Tutoring
starts at
per month
A personal study tutor helps you achieve your goals in regular 1-on-1 tutoring sessions.
Frequently Asked Questions about the MCAT exam
The Medical College Admission Test (MCAT) is a standardized, multiple-choice exam that is required for admission to medical schools in the USA and Canada. The test assesses problem-solving, critical thinking, and knowledge of natural, behavioral, and social science concepts and principles prerequisite to the study of medicine.
The MCAT is divided into four sections: Biological and Biochemical Foundations of Living Systems; Chemical and Physical Foundations of Biological Systems; Psychological, Social, and Biological Foundations of Behavior; and Critical Analysis and Reasoning Skills.
Each of the four sections of the MCAT is scored from 118 to 132, with the mean and median at 125. This means the total score ranges from 472 to 528, with the mean and median at 500.
The amount of study time needed for the MCAT can vary greatly depending on your background knowledge and test-taking skills. A common recommendation is to spend at least three to six months preparing for the MCAT.
You can take the MCAT up to three times in a single testing year, four times in a two consecutive-year period, and seven times in a lifetime.
Ideally, you should take the MCAT in the calendar year prior to the year in which you plan to enter medical school. This allows ample time for score reporting before application deadlines.