Playlist
Show Playlist
Hide Playlist
Oxygen Transport – Lung Physiology
-
Slides 02 LungPhysiology Basic.pdf
-
Download Lecture Overview
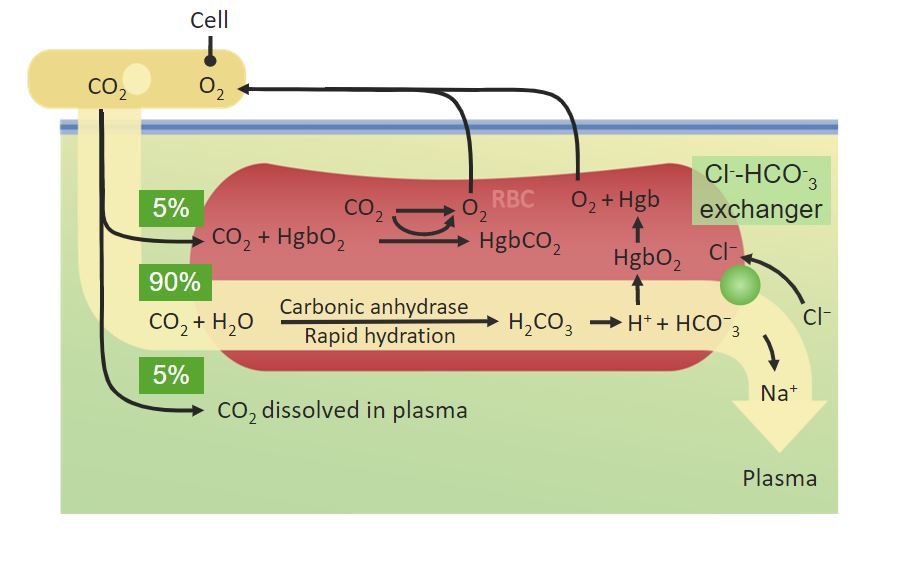
00:01 So the next is we describes so far for ventilation and oxygen uptake by the blood are really very simple - ventilation just requires a little bit of expansion of the lung, oxygen uptake is diffusion -- very straight forward simple mechanisms. 00:16 Oxygen transport by the blood is a little bit more complex and there are two main forms by which oxygen that reaches the blood can be transferred using that blood to the tissues to oxygenate the body. 00:30 One is to dissolved oxygen that’s measured by doing the arterial PaO2 level, that actually only represents a very small amount of the total oxygen in the blood, one or two percent. It represents the oxygen is dissolved in the liquid phase of the blood. 00:46 The majority of oxygen transport in the blood is via the red cell and that is because the red cell is packed full of hemoglobin and each hemoglobin molecule can carry four oxygen molecules. 00:59 They're about 250 million hemoglobin molecules per red cell and red cells is by far the most dominant cell present in the blood, so that provides a massive oxygen carrying capacity, so the oxygen picked up by the blood in the lungs is transferred to the hemoglobin and then using the red cells being transported by the blood through the left side of circulation and into the systemic arterial circulation will reach the tissues and deliver oxygen to where it is needed - in the muscles, the brain, the kidneys, etc. 01:30 which are working and require the oxygen for their aerobic metabolism. 01:34 The saturation of hemoglobin, how much oxygen it is carrying, is measured using a pulse oximeter probe which you can just place on somebody’s finger, or their toe, or their ear, and will give you a feel for whether the hemoglobin has it’s maximal oxygen saturation or the oxygen saturation is low. 01:55 So the total amount of oxygen delivery to the peripheral tissues is depending on a few factors. 02:02 One, is the oxygen content of the blood and that’s dependent as we’ve already mentioned on hemoglobin largely and to certain extent on the PaO2, how much oxygen is dissolved in the liquid phase of the blood. 02:14 But in addition, it depends on cardiac output how much blood is being delivered to the tissue, so that is why a cardiac function is very important for patients with breathlessness - a significant component of how oxygen gets to peripheral tissues. 02:32 If you look at the curve, in the lung tissue, we get left shift of the curve so there’s higher affinity of hemoglobin for oxygen and that occurs because of the relatively low con - dioxide concentration - the relatively low temperature present there and the relatively non-acidotic conditions found within the lung. 02:54 In the tissue, the opposite occurs. Metabolizing tissue will cause acidosis because it produces hydrogen ions, there'll be CO2 present because of the metabolism as well, there'll be temperature being produced by the active cells and in addition there's a compound called 2,3-diphosphoglycerate, 2,3 DPG which has high concentrations within skeletal tissue but low concentrations in the lung and that helps cause the shift to the right, to release of oxygen in metabolizing tissue. 03:28 So this is how the affinitive hemoglobin for oxygen can be manipulated so that actually is able to take up oxygen in the lungs but deliver it to tissues which are metabolizing. 03:41 Carbon dioxide is also transported by the blood but there are three different forms here, rather than just the two for oxygen. 03:50 Like oxygen you can dissolve carbon dioxide into the blood and that actually makes a significant contribution towards carbon dioxide delivery from metabolizing tissues back to the lung about 8%. 04:05 It is also can be attached like oxygen to hemoglobin but that’s a relatively weak process for carbon dioxide compared to oxygen and only about 11% for carbon dioxide has been transferred from the tissues back to the lungs is in the form of carbaminohemoglobin attached to deoxygenated hemoglobin. 04:23 The majority of carbon dioxide transport from the tissues back to the lungs is as dissolved bicarbonate. 04:28 So how does carbon dioxide become bicarbonate? Well, this is a reaction that is catalyzed by an enzyme called carbonic anhydrase, mainly occurring in red blood cells. 04:37 Carbon dioxide plus water is converted by carbonic anhydrase into a carbonic acid and that quickly dissociates to form bicarbonate and hydrogen ions. 04:46 And this is why carbon dioxide increases the concentration of hydrogen ions and therefore can cause acidosis. 04:52 There are a couple of effects of this. 04:55 A hydrogen ion binding to hemoglobin lowers its affinity for oxygen as called the Bohr Effect as I’ve mentioned before that’s one of the main mechanism that makes the oxygen dissociation cause shift to the right and releases oxygen for the tissues to use. 05:10 Oxygen binding to hemoglobin reduces the affinity of carbon dioxide and that’s called the Haldane effect and so you end up with the reverse occurring in the lungs while oxygen binding to hemoglobin actually releases carbon dioxide and allows back excretion of carbon dioxide. 05:27 So carbon dioxide and acid base are very closely related. 05:36 The pH of the body is actually very tightly regulated. 05:41 The normal pH varies between 7.36 and 7.45. You can just about tolerate pH’s 6.8 and 6.9, but essentially I haven’t seen many people who have survive to have pH's that low to respiratory acidosis. 05:55 A metabolic or respiratory alkalosis can push your pH as high as 7.7 and that reflects a hydrogen ion concentration over an 8 fold range of 160 to 20 nmol per liter. 06:07 So how is pH regulated? Well, it’s by getting rid of acid and the way we get rid of acid is almost 100% through the lungs 99% and that’s a rapid method of controlling pH. 06:22 The kidneys can also get rid of acid but they are much slower responder and that takes hours and days to actually respond to an increase in hydrogen ions so largely, it’s a lung control situation. 06:35 You can also buffer acid, so if there's an excess of hydrogen ions present, buffers are substances that will pick up this hydrogen ions and make the acidosis less of a problem, reduce the pH effects. 06:48 And the key buffers that do that is bicarbonate, because if you have bicarbonate and you add hydrogen ions there then that will convert it to carbon dioxide and water and you can breathe out the carbon dioxide and water of course is a safe substance, and again that’s a process that is catalyzed by carbonic anhydrase, it’s one of those enzymes that can catalyze that process in either direction. 07:12 Another key buffer is hemoglobin. 07:15 As deoxygenated hemoglobin has a negative charge and that allows it to attract some of the positive charged hydrogen ions and buffer some of the effects of excess acid. 07:25 But largely speaking, bicarbonate is the most important buffer. 07:28 There are other components of the blood which can act as buffers as well. 07:32 So, this is a slide that describes an overview of acid-base balance and the lungs are very central to control the pH because of this - this is about carbon dioxide cuz pH is largely dictated by the concentration of carbon dioxide and bicarbonate. 07:49 So on the left we have tissue production or ingested hydrogen ions that causes a degree of acidosis. 07:56 That acidosis means that bicarbonate is converted to carbon dioxide and water and that carbon dioxide is breathed out by the lungs getting rid of that excess acid. 08:08 So if you have a problem with respiration, hypoventilation that will lead to respiratory acidosis because you’re not able to get rid of the carbon dioxide and that means you’re not able to get rid of the acid that’s being produced by the body’s metabolism. 08:23 Importantly, if you have an acidosis due to poor respiration, if you increase the amount of bicarbonates around that acts as a buffer and can helps control some of the problems you might get with the excess hydrogen ions so there’s compensation occurs in people with long term under-ventilation of the lung over hours and days, you’ll get an increase in bicarbonate to compensate for that respiratory acidosis. 08:49 Conversely, if you breathe very fast for no particularly good reason, hypoventilation, then in fact you’ll blow off a little carbon dioxide and that will drive up the pH and cause a respiratory alkalosis. 09:02 Over courses of acid-base imbalance, well, the most importantly one is excess acid production most classically that happens in diabetic keto-acidosis and in renal failure, where there's a lot of extra acid being produced and you require the lungs to ventilate faster to try and get rid some of the extra acid. 09:21 There's an equation called the Henderson-Hasselbach Equation which dictates pH and if you - this is the equation right in here, pH equals 6.1 plus log by carbonate concentration divided by the log point 0.03 of the PCO2. 09:40 Now what this really means is that the pH is dependent on the bicarbonate and the carbon dioxide concentration. 09:47 The carbon dioxide concentration is set by the lungs, the bicarbonate is largely set by the kidneys so when you get your respiratory acidosis and compensation will increase bicarbonate that is because the kidneys are retaining more bicarbonate. 10:00 If you do a blood gas on somebody with an acid-base imbalance due to the lungs who have respiratory acidosis, under ventilation, you get a low pH, acid; a high PaCO2 because this issue, the problem here is you’re not able to get rid of the CO2 and that’s driving the acidosis and in compensation over time, over a day or two, you’ll get a positive base excess which represents the amounts of buffer available within the system, a positive base excess means there's a lot more buffer than normal and a high bicarbonate as we’ve discussed just now. 10:34 And respiratory alkalosis is the reverse occurs, you get a low - if you breathed too fast you get a high pH, a low PCO2 and the bicarbonate gets blown off by the hypoventilation which is why you’re getting alkalosis in the first place. 10:48 So now we'd move on to describe the pulmonary circulation. 10:55 Now what’s important here is how much blood is being delivered to each alveoli and what we mean by that is that if you have an alveolus that is not being ventilated for whatever reason, then if you deliver blood to the alveolus it won't be oxygenated cuz there’s no oxygen in the alveoli units and that is called VQ miss matching and the opposite can happen. You can have blood not being delivered to an alveolus and that alveolus is being ventilated and that means that that alveolus ventilation actually has no functional consequences, there’s no blood being delivered so no oxygen can be taken up by the blood and this occurs in different parts of the lungs to a different extents. 11:38 So for example, in the normal lung, there’s relative under-perfusion of the lung apices. 11:43 There’s less blood being delivered compared to how much ventilation is occurring, whereas at the bottom of the lungs it’s the other way around, more blood is being delivered compared to the amount of ventilation that is being delivered, so that’s a normal physiological situation where there’s a little bit of VQ miss matching occurring. 11:59 Another very important point here is that the pulmonary arteries constrict and response to the oxygen concentration or if it’s a - so, if you have a low oxygen concentration, you get a pulmonary artery constriction occurring and that allows the amount of blood being delivered to be diverted away from parts of the lung which are not ventilating very well so if you got a bit of lung that is not delivering oxygen to the alveoli then there’s no need -- there’s no requirement for blood being delivered to that area and in fact the pulmonary arteries will constrict in response to the hypoxia that’s occurring at that part of the lung, and that’s fine because that allows the ventilation perfusion match into and to occur as much as possible within the normal lung, the problem with that is that if you have a chronic lung disease where everywhere within the lung is hypoxic essentially, then you end up with pulmonary artery constriction reoccurring throughout the lung and that leads to pulmonary hypertension and cor pulmonale, which is the end stage problem that occurs in many patients with chronic lung disease. 13:03 High blood pressure in the pulmonary artery circulation lead into the right sided cardiac failure and all down to the fact where you're getting the hypoxic vasoconstriction, that’s a normal physiologic response in a normal lung but in a pathological circumstance can have negative consequences. 13:20 The control of ventilation. 13:25 Now, ventilation is controlled mainly by the back of the brain: the medulla, the pons, and the brain stem. 13:33 There's an inspiration center in the pons so that basically drives the ventilation process that we described earlier with diaphragmatic contraction, intercostal muscle contraction leading to increased size of the lungs, about -- a rate of about 12 per minute so that’s your normal resting ventilation that requires no effort and occurs automatically. 13:54 Expiration is normally a passive process when you're at rest but if you’re exercising it becomes a more active process with contraction of the intercostal being relevant and potentially the abdominal muscles as well and therefore there is an expiratory center which is in the medulla that works mainly during forced expiration when you’re exercising or if you have chronic lung disease and have to respire over greater rate than normal. 14:20 How does the brain responds to what’s the environments work out whether to breathe faster or slower? And that’s largely driven by chemoreceptors which sample with the blood peripherally in the aortic arch, the carotid arteries, and the bifurcation across the arteries and centrally in the medulla. 14:39 And what these response to is evidence of respiratory acidosis, so the peripheral receptors will measure the carbon dioxide concentration and if that’s increased or the pH is decreased suggesting a degree of mild respiratory acidosis, then they will drive respiration up. 14:57 They also respond to oxygen but to a lesser extent that has to be quite mark changes in the oxygen concentration of the blood to drive respiration up. 15:06 The central receptors are largely driven by pH but that’s as we’ve discussed already is basically dependent on the concentration of carbon dioxide in the blood. 15:16 So what happens here is that if for example, you are working harder, you’ve decided to run for a bus that would drive a more conducts - production by your skeletal muscle that would decrease your pH and perhaps your pH might fall a little bit as well. 15:36 This will be detected by the peripheral and central chemoreceptors and that will drive increased central respiratory drive and then you’ll fire your phrenic nerve and your intercostal nerves more; you’ll ventilate faster and that will overcome the mild degree of acidosis generated by your quick burst of activity and then the opposite would occur if you’re resting or you're being inactive with decreased ventilation occurring as the pH comes back up again and the CO2 goes down.
About the Lecture
The lecture Oxygen Transport – Lung Physiology by Jeremy Brown, PhD, MRCP(UK), MBBS is from the course Introduction to the Respiratory System.
Included Quiz Questions
On average, how many hemoglobin molecules does an erythrocyte contain?
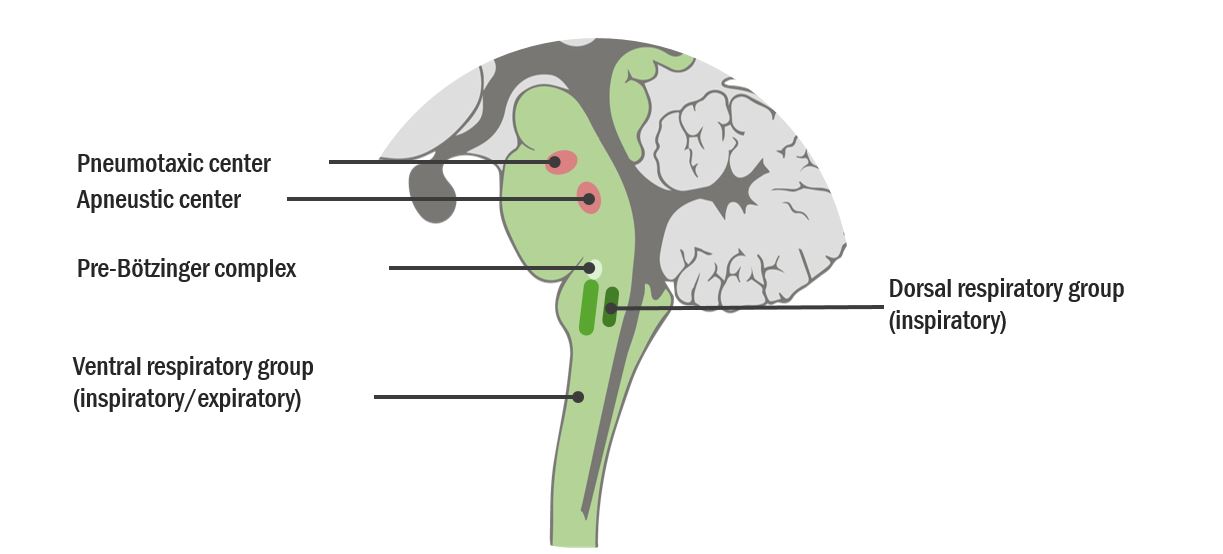
- 250 million
- 50 million
- 100 million
- 140 million
- 200 million
Which of the following is the most abundant cellular component of blood?
- Erythrocytes
- Leukocytes
- Neutrophils
- Eosinophils
- Macrophages
In which of the following structures can pulse oximetry be performed?
- Ear lobe
- Radial artery
- Aorta
- Abdomen
- Chest
Which of the following will cause a left shift in the oxygen dissociation curve?
- An Increase in pH
- An elevated PCO2
- A decrease in pH
- An increase in temperature
- A decreased PO2
What proportion of CO2 is dissolved in the blood?
- 8%
- 81%
- 11%:
- 6%
- 10%
The concentration of bicarbonate in the blood is mainly regulated by which organ(s)?
- The kidneys
- The lungs
- The spleen
- The blood itself (it can self-regulate)
- The skin and soft tissues
You receive a call from your ward that one of your patients is breathing "very little." During the physical examination, you notice decreased excursions of the chest and bradypnea and request blood gases to be taken. What pH value would indicate that your patient has respiratory acidosis?
- A pH < 7.36
- A pH < 7.12
- A pH > 7.22
- A pH > 7.26
- A pH < 7.32
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |