Playlist
Show Playlist
Hide Playlist
Blood pH: Acidosis & Alkalosis – Acid Base Balance
-
Slides 07 pHBuffers AcidBaseBalance GeneralPhysiology.pdf
-
Download Lecture Overview
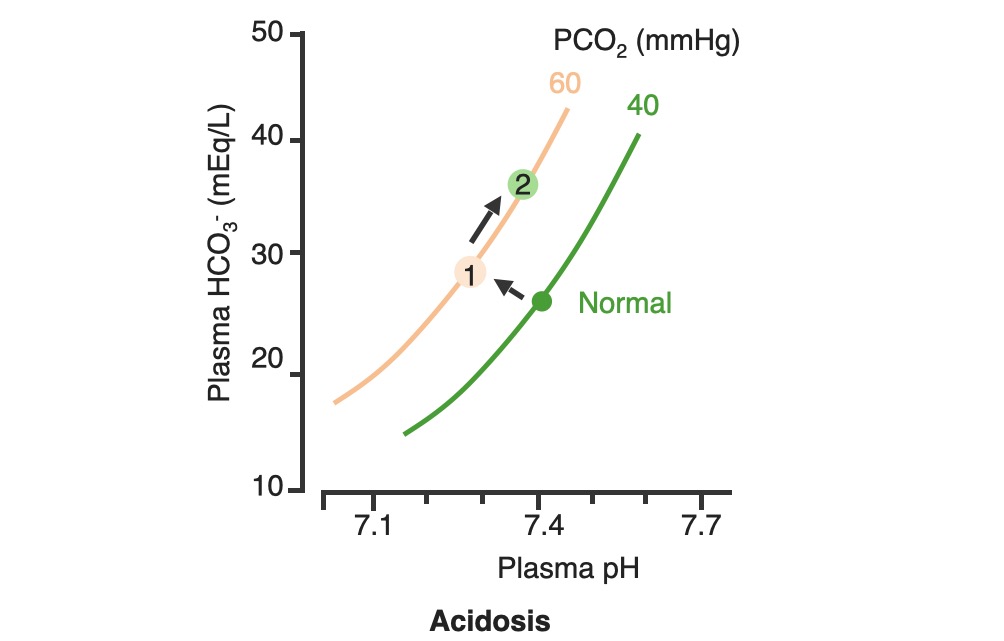
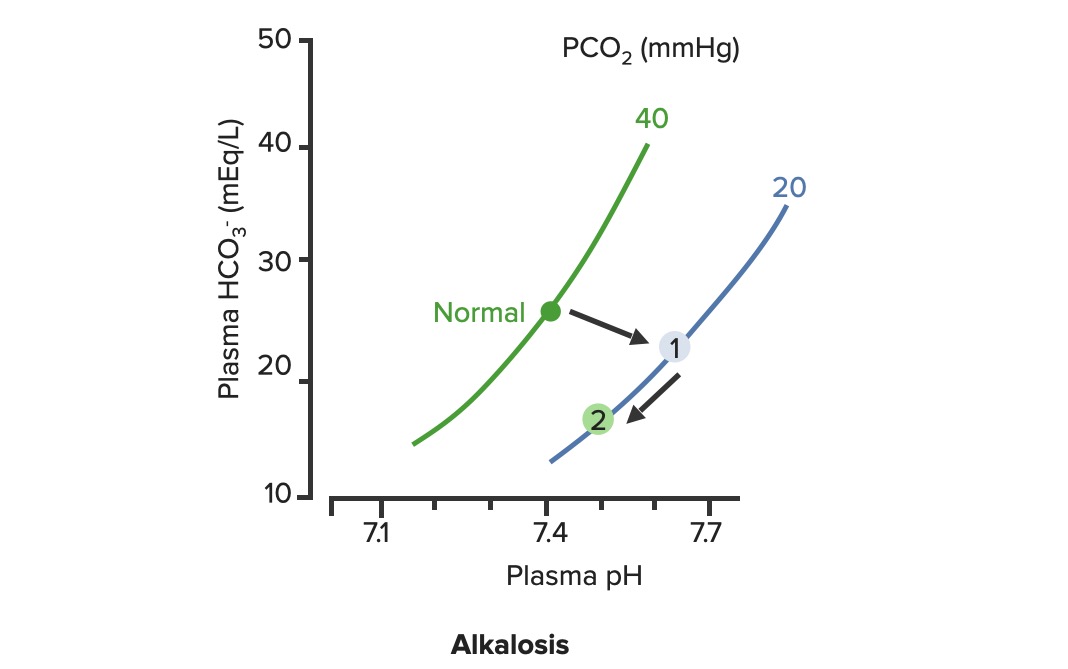
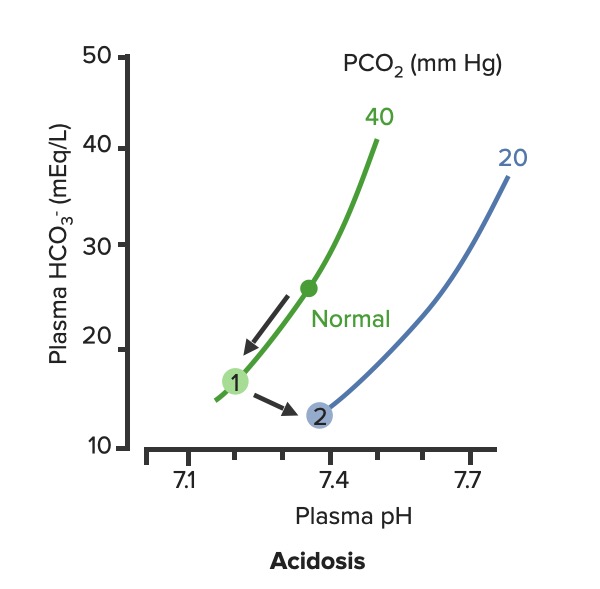
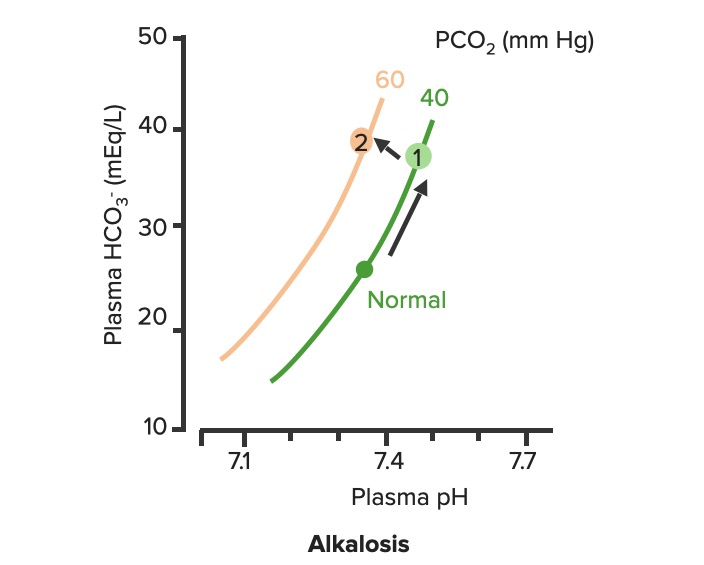
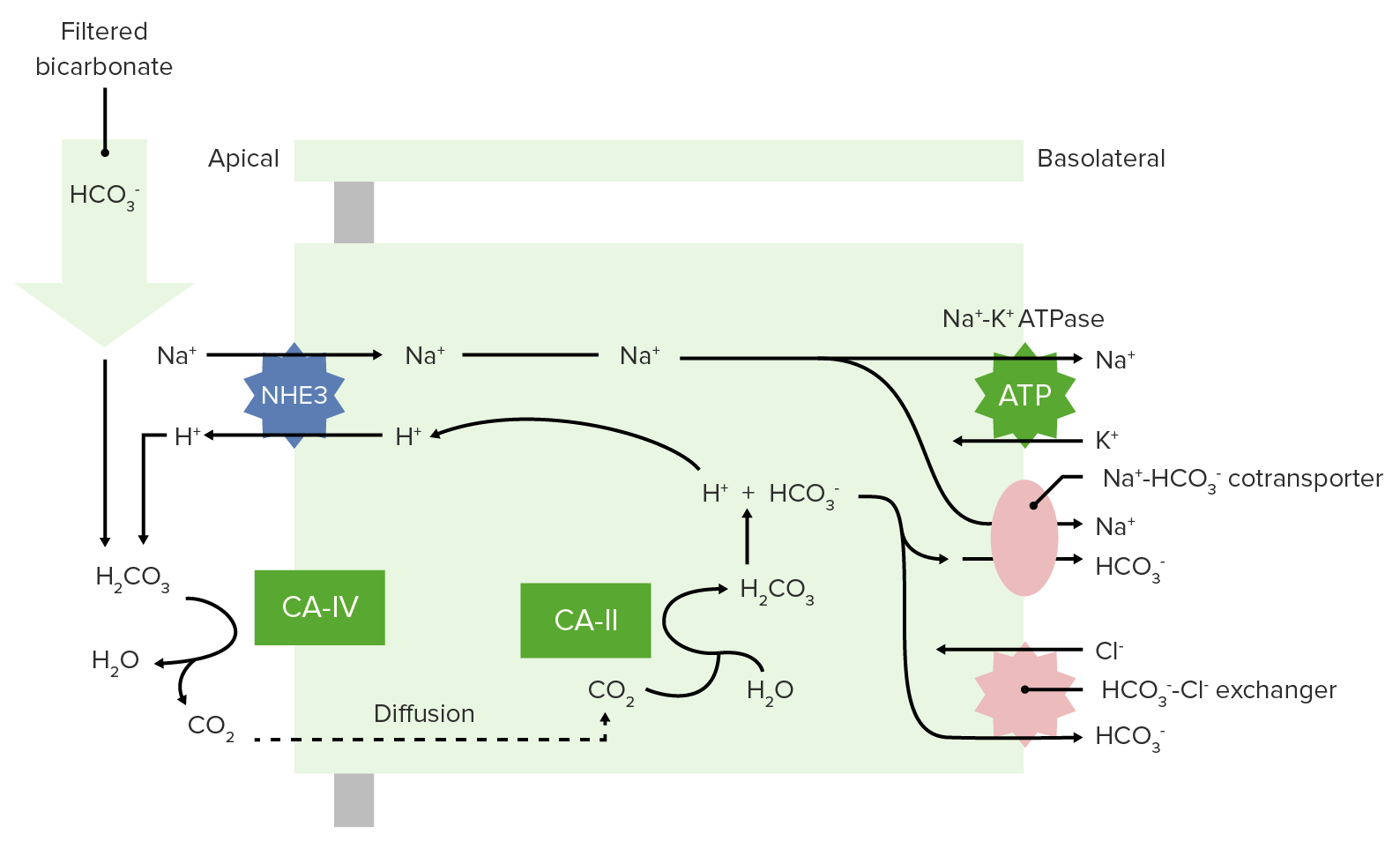
00:00 So let’s go with blood pH as the next thing we’re gonna discuss. 00:05 Blood pH is so very important because blood is gonna be delivered to each cell in the body. 00:12 Therefore, if blood pH is off, you could alter the pH of a lot of tissues throughout the body. 00:18 Normally, blood pH be somewhere between 7,35 and 7,45. 00:24 So let’s go see some examples of what changes happen with alterations in blood pH. 00:30 If you add hydrogen ions to the blood, you get an acidemia. 00:35 So an acidemia is a pH less than 7,35. 00:39 So if you want to look at these in terms of how much acid is added. 00:44 Let’s just give an example of more of our acidic gnomes. 00:48 What’s that gonna do is tilt the pH down to a lower level. 00:54 If you think about reducing the amount of hydrogen ions, these now becomes more basic and you have an alkalemia. 01:04 So this is a pH above 7,45. 01:08 Be think about this in terms of our tutor tauter example, it means that you have more basic components than you have acidic components which tilt the scale over this direction. 01:22 Therefore, really we have two different conditions we needed to focus on. 01:26 We have acidemia and alkalemia. 01:28 The emia, the -ie portion, the -mia portion of the alkalemia that is saying where it is problematic such as the blood. 01:40 When you look at blood pH, this kind of scale work very well to be able to emphasize, how many more hydrogen ions you have at low pH as compared to high pH is. 01:52 So in this case, that we have blood pH on the X-axis and we have hydrogen ions on the Y. 01:57 You can see this log rhythmic scale. 02:00 if you traumatically increase the number of hydrogen ions, you reduce the pH. 02:07 As you have a decrease in the amount of hydrogen ions, then you an increase in pH. 02:13 You wanna think about, where though the hydrogen ions come from? This is gonna be a very important component when trying to diagnose someone’s acidemia or alkalemia. 02:24 Where are the hydrogen ions from? So always ask yourself that. 02:31 The second thing is, is we are gonna be able to buffer some of those hydrogen ions. 02:37 To buffer something means that you will bind it to another molecule. 02:42 You take it out of concentration. 02:46 It doesn’t mean that the hydrogen ion has gone away. It just means that you’ve bound up. 02:50 In that case, you wanna think about, where are the buffers? And finally, if there are any basis that were added to the system, you’ll think of where are they coming from? So if you always think about, okay, where the hydrogen ions coming from? Where are the bases is coming from? Where is the HCO3- coming from? You’ll be able to help your diagnostic procedure to be able to figure out, why you have an acidemia or an alkalemia?
About the Lecture
The lecture Blood pH: Acidosis & Alkalosis – Acid Base Balance by Thad Wilson, PhD is from the course Acid-Base Balance.
Included Quiz Questions
What is acidemia?
- A blood pH of less than 7.35
- A tissue pH of less than 7.35
- A blood pH of more than 7.35
- A tissue pH of more than 7.35
- A blood [H+] of less than 0.0000735
Why is it important that blood pH remains within tight parameters?
- An altered blood pH affects the entire body.
- Blood feels the effects of the pH of the entire body.
- Cells regulate blood pH.
- An abnormal blood pH cannot be buffered.
- There are no compensatory mechanisms to restore the pH toward normal.
What are buffers?
- Substances that bind to hydrogen ions and decrease the number of free protons in the solution.
- They are always bicarbonate.
- Substances that change the pH from acidic to basic.
- Substances that remove the hydrogen ions from the blood.
- Substances that remove the hydrogen ions from the tissues.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
1 customer review without text
1 user review without text