Playlist
Show Playlist
Hide Playlist
Metabolic Acidosis & Alkalosis
-
Slides 09 ToolsCompensation AcidBaseBalance GeneralPhysiology.pdf
-
Download Lecture Overview
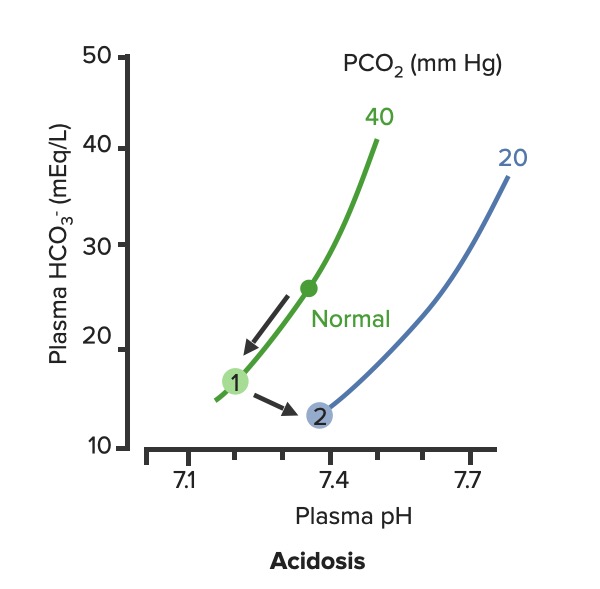
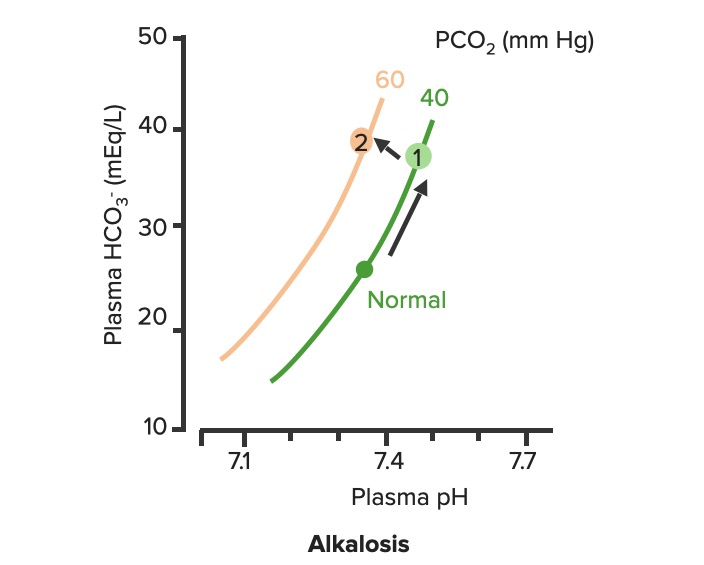
00:01 Okay, what are our few ways that you can obtain a metabolic alkalosis? A few of them are, if you had too much bicarb. 00:09 So you can have too much bicarb if you overdose on an Antacid. 00:13 Another way you could get too much bicarb is by losing too many hydrogen ions. 00:20 And this can happen in prolonged vomiting over a number of days or if you had a nasogastro tube and you were suctioning out your stomach contents. 00:31 All of those would increase your pH and increase your bicarb levels. 00:39 Okay, let's take a look at this on the diagram. 00:43 Again, we have pH, we have bicarb and we have the isopleth on the PCO2 line. 00:51 If we have an increase in bicarb and an increase in pH, you know which directions you're going to travel. 00:59 In this case, we're going up the PCO2 line. 01:03 So, you're going from normal, and normal again is at pH of 7,4, a bicarb level of 24, and PCO2 of 40. 01:12 You're travelling in the upward direction and that gives you a higher bicarb level and a higher pH. 01:24 So, you want to think conditions associated with this are either ones that you gained too much bicarb or lose too much hydrogen ions. 01:37 Either way you can get to the same point. 01:40 You may ask "Well, how would you know the difference"? On a diagram you won't know, that's where you need your patient history to get more information about which one will be driving this particular response. 01:53 Okay, let's now look at metabolic acidosis. 01:59 There are a lot of different types of metabolic acidosis. 02:02 So, we have to go through each one of them step by step. 02:07 You could have too much acid production, through things like lactic acidosis, this means you're gone through anaerobic metabolism and you're producing too many hydrogen ions. 02:17 How do you do that' You go down glycolysis to pyruvic acid. And then, from pyruvic acid, instead of going through the Krebs cycle, you go through Lactate dehydrogenase reaction, and form lactic acid. 02:31 You do that when you need energy quickly. 02:33 You don't have time to wait to go through the whole Krebs cycle. 02:37 You need to get energy right now, to do that you might make lactic acid. 02:42 Another way is through ketoacidosis. 02:45 So, a diabetic often times you can't use glucose or go through ketoacids to get enough metabolism. 02:52 What are other ways you can have a metabolic acidosis? Well, some of them are related to poisons that you might take in. 02:59 And this could be accidental or this could be on purpose. 03:03 You could have propylene glycol being consumed. A wood alcohol, even overdose you know, on certain drugs like aspirin. 03:12 You could also have large amounts of bicarb loss. 03:16 And that would be something like losing a lot of dietary contents such as in diarrhea. 03:23 So, in this case, you have a lot of bicarb that's being pumped into the GI system from the pancreas. 03:29 If you keep losing that out of the body, you will continually to dump a bicarb. 03:35 Type II - Renal Tubular Acidosis also causes a bicarb loss. 03:40 The final way that you can have a metabolic acidosis is either through a Type I or Type IV Renal Tubular Acidosis. 03:48 These are both acid secretion issues. 03:53 So, knowing that there are four different mechanisms that cause a metabolic acidosis, we're going to have to try these through which is which. 04:01 and we have a couple of extra tools to do so. But let's talk to those in just a minute. 04:08 First, let's go back to the diagram and figure out how a metabolic acidosis looks on one of these diagrams. 04:17 Again, we start off at normal. Normal as a PCO2 of 40, a pH of 7,4 and a bicarb of 24. 04:26 In metabolic acidosis is traveling down the PCO2 line. 04:33 This causes a decrease in pH and a decrease in bicarb always. 04:42 If you have a metabolic problem, how do you respond to it? Usually, respond with the opposite system such as a respiratory compensation to a metabolic problem. 04:53 Okay, let's go through these two problems with their appropriate compensations. 04:59 A metabolic alkalosis is fixed from a respiratory acidosis. 05:07 So, in this case you go from normal up to PCO2 line to number one. 05:13 And then, from number one to number two. 05:17 Number two should bring pH back closer to normal. 05:22 If it fully compensates, if it brings it back to the original pH or to within the original pH range, This is a nice response. 05:32 So, you have a problem with the metabolic alkalosis and how does the body respond to that? It decreases alveolar ventilation which increases CO2. 05:46 If you have the opposite problem, which is a metabolic acidosis, you will travel down the PCO2 line. So, this causes a lower pH and a lower bicarb. 05:58 How do you try to fix this problem? By breathing more often. 06:04 You will now breathe out more CO2 and as you breathe out more CO2, pH will start to return towards normal. 06:15 You noticed here that we are traveling along kind of a linear sloped line from one to two in both of this conditions. 06:25 That is because you always have to travel between this isopleths along the blood-buffer line.
About the Lecture
The lecture Metabolic Acidosis & Alkalosis by Thad Wilson, PhD is from the course Acid-Base Balance.
Included Quiz Questions
If respiratory compensation is occurring in response to metabolic acidosis, what is the predicted change in PCO2 levels?
- Large decrease in PCO2
- Small increase in PCO2
- Large increase in PCO2
- Small decrease in PCO2
- No change in PCO2
Which of the following laboratory results are indicative of compensated metabolic alkalosis?
- High pCO2, high bicarbonate and pH normal or slightly elevated
- Low pCO2, low bicarbonate, low pH
- Low pCO2, high bicarbonate, and high pH
- Low p CO2, normal bicarbonate and, high pH
What is the most likely diagnosis in a woman suffering from insulin-dependent diabetes mellitus with a pH of 7.2, HCO3-17 mmol/L, and pCO2-20 mmHg?
- Metabolic Acidosis
- Metabolic Alkalosis
- No acid base imbalance
- Respiratory Acidosis
- Respiratory Alkalosis
Which of the following statements regarding the renal handling of acids in metabolic acidosis is FALSE?
- Bicarbonate reabsorption is decreased.
- Hydrogen ion secretion is increased.
- Urinary acidity is increased.
- Urinary ammonia is increased.
Which of the following statements regarding metabolic alkalosis is TRUE?
- It can be caused by vomiting and loss of hydrogen ions.
- It is associated with a decrease in pH.
- It can be caused by a loss of bicarbonate.
- It can be caused by an impaired acid secretion.
- It is caused by a gain of hydrogen ions.
A child with acute asthma has a PaCO2 of 48 mmHg, a pH of 7.31, and a normal HCO3 blood gas value. Which of the following is the most likely diagnosis?
- Respiratory acidosis
- Respiratory alkalosis
- Metabolic acidosis
- Metabolic alkalosis
- Normal
Customer reviews
4,0 of 5 stars
| 5 Stars |
|
0 |
| 4 Stars |
|
1 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
On the slide about respiratory compensation for metabolic acidosis, the compensation slope with decrease Pco2 level has a PCO2 value of 60 which should be 20. Becauase in respiratory Compensation for MA PCO2 slightly decreases That is just a typo, but can been confusing The lecture itself is very good