Playlist
Show Playlist
Hide Playlist
Cancer Drugs & Chemotherapeutic Agents: MTX, 5-FU, 6-MP, 6TG & Ara-C – White Blood Cell Pathology
-
Slides Leukemia White Blood Cell Pathology.pdf
-
Download Lecture Overview
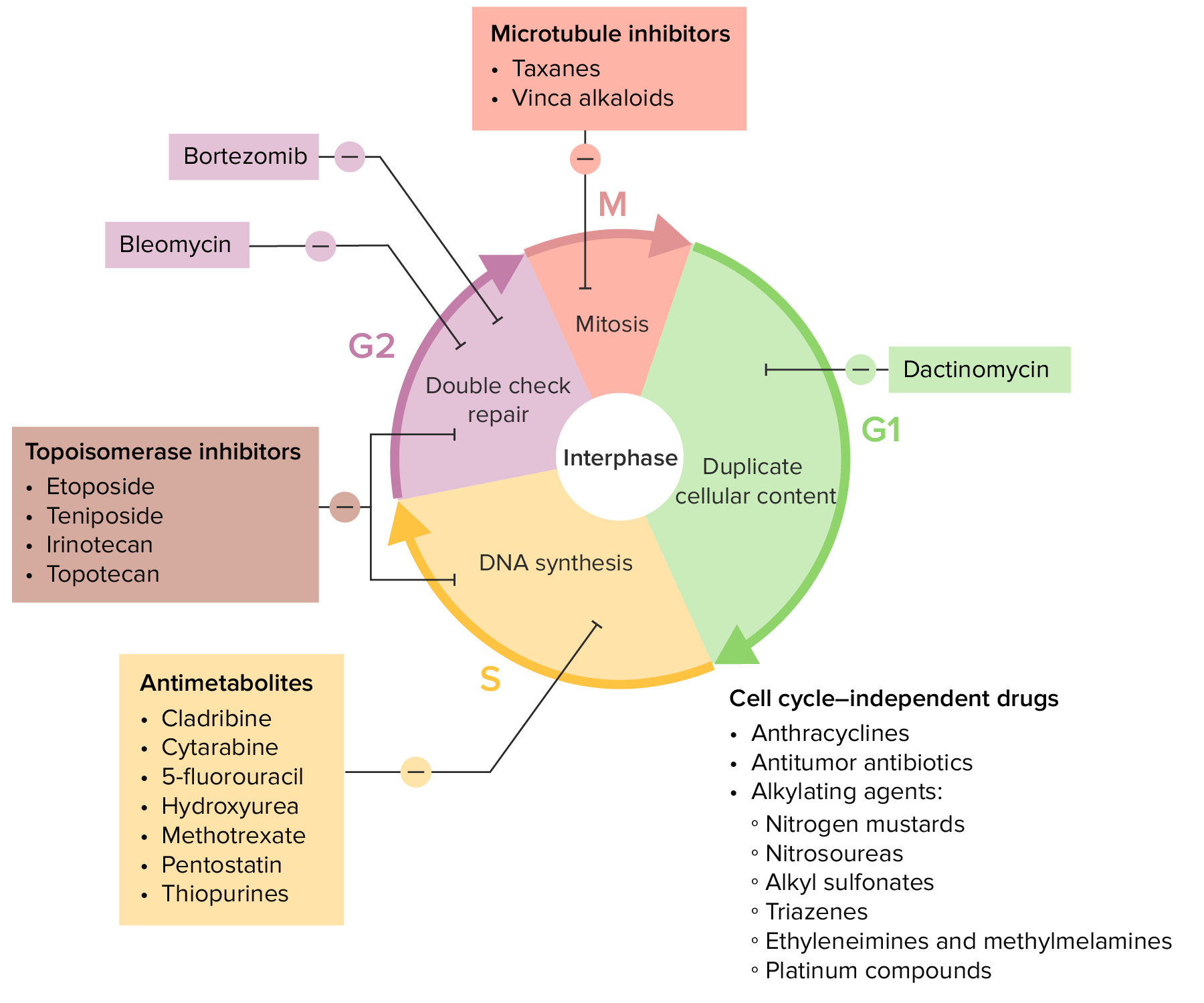
00:00 Let’s take a look at the most important arrest point, between G1 and S phase. 00:04 There’s a nice little cycle here to show you where your antineoplastics will be located. 00:11 Let’s begin. 00:13 If you’re looking at your S phase, which is DNA synthesis replication, that will be antimetabolites. 00:18 And I’ll walk you through tables. 00:19 There are tables and tables and tables that are coming. 00:22 They’re all beyond valuable. 00:25 I mean, they’re just absolutely invaluable, okay? Now, you know the tables well. 00:29 There’s no way you can miss a question in pathology or pharmacology about cancers, seriously. 00:34 So, let’s take a look. 00:35 But first, organize your thoughts. 00:37 Antimetabolites, DNA replication has been inhibited. 00:41 Then you have a drug called etoposide. 00:43 Etoposide, when I walked you through the mechanism of action, well, not only inhibits a neoplastic cell. 00:49 Remember, how long does a cancer cell want to be in here? Obviously, forever. 00:54 Where am I? In the cell, in the nucleus, right? Because I’m trying to -- DNA mitosis. 01:00 So now, etoposide, S and G2 phase will be inhibited. 01:04 What does G2 mean to you? Post-translational modification. 01:09 And bleomycin specifically works in G2. 01:12 And then in your M phase, which is your mitosis. 01:16 Okay, now, of all the mitosis in pathology, you’ll be paying attention to meta, meta, metaphase; prophase, metaphase, then you have your anaphase, telophase, right? The point is your metaphase is when the chromosomes will be lined up in the middle. 01:31 That’s important. 01:32 And those of your vinca alkaloids and what’s known as your taxols. 01:39 Now, this is your dogma for your DNA synthesis. 01:44 So we’ll take a look at nucleotide synthesis and their inhibitors, such as methotrexate, 5-FU, a decrease of thymidine synthesis, and then you have other drugs such as 6-mercaptopurine, which then knocks out your purine. 01:58 Then we go to DNA, and these will be alkylating agents. 02:00 We’ll talk about cisplatin, cross-linking will be important as what they do. 02:05 They cross-link to your DNA. 02:07 Then you have dactinomycin and doxorubicin. 02:10 Doxorubicin, we’ve talked about in cardiology because it brings about what’s called as dilated cardiomyopathy. 02:15 This will intercalate the DNA. 02:18 Then you have etoposide, and this is a drug that knocks out your topoisomerase II. 02:22 If I were you, I’d go ahead and memorize the enzyme now, if you haven’t already, topoisomerase, etoposide. 02:31 On the side of cellular division, we’ll take a look at vinca alkaloids, which inhibits microtubule formation, and paclitaxel, which then inhibits microtubule disassembly. 02:40 Before we move on, can you think about when cellular division takes place in your cell cycle? MMM phase, and you could perhaps inhibit the division by two ways. 02:51 Either you never create a spindle to split the chromosome, or number two, you formed the spindle but then you froze it. 03:00 So maybe you have a freeze gun and you freeze your -- you’re the fire extinguisher and you then freeze your tubules. 03:10 They will not divide any further. 03:12 Could that be any more dramatic for you? Cellular division, vinca alkaloids and paclitaxel, you’re inhibiting, taxane. 03:22 Our first drug, methotrexate. 03:24 Mechanism of action, it inhibits your dihydrofolate reductase. 03:29 That’s important. 03:30 If you inhibit your dihydrofolate reductase, you will not be forming the important thiamine that you require for proper DNA synthesis. 03:40 What kind of cancers might you be looking for? Leukemias, choriocarcinomas, and your sarcomas. 03:48 Non-neoplastically, big time, look for rheumatoid arthritis. 03:51 You’re looking at this being an immunomodulator. 03:53 Therefore, psoriasis, and you’re looking for your rheumatoid arthritis. 04:01 Now, the toxicity is the following. 04:03 Only for cancer, by the way. 04:06 Myelosuppression, high dose. 04:08 High dose. 04:09 So what may happen here? Do remember I was talking about methotrexate with what’s known as your megaloblastic anemia? And the fact that you inhibit dihydrofolate reductase, it means that you won’t be able to properly recycle your folate. 04:22 You’re going after cancer cells. 04:24 Uh-oh, there’s war that’s being waged. 04:28 There’s always innocent people that are being harmed. 04:31 The innocent cells that are being harmed here are your cells. 04:36 Your normal cells are being harmed and they’re being killed. 04:40 You don’t want that. 04:42 So, with myelosuppression, you’re then going to rescue your patient. 04:47 You’re going to rescue those normal cells. 04:49 You’re going to fly in those helicopters so that you can then nourish your normal cells. 04:55 Welcome to leucovorin, folinic acid. 04:59 Why doesn’t it say folic acid? I’m sorry, but if you’re giving methotrexate, you’re trying to kill the enemy, which is the cancer. 05:06 As you continue giving folic acid to your cancer cells, how’s that going to help? So, you’re specifically giving folic acid derivative known as folinic acid only for your normal eukaryotic cell. 05:20 How ingenious is that? But that’s only for high doses, cancer. 05:26 You won’t be giving that if you’re thinking methotrexate and rheumatoid arthritis. 05:29 You wouldn’t be giving such high doses. 05:31 It’s called macrovesicular fatty change in the liver, and mucositis, and there’s absolutely teratogenic for your fetus. 05:39 Important toxicities. 05:41 Welcome to methotrexate. 05:44 5-FU, 5-fluorouracil, as a pyrimidine analog, bioactive. 05:49 It behaves like your bioactivated 5-FdUMP. 05:54 This is when your dUMP wants to become your dTMP. 05:58 he name of the enzyme here is called thymidylate synthase. 06:01 So therefore, here once again, dTMP is decreased in the cancer cell. 06:05 It decreases your protein synthesis. 06:08 The biggest difference with this is the fact that leucovorin will never come in handy. 06:12 In fact, many oncologists use leucovorin with FU to enhance its activity maybe for treating colorectal cancer. 06:22 But there’s another drug that you need to know that you’ve learned about in pharm and that your monoclonal antibody known as cetuximab for colorectal cancer. 06:30 Also, synergistic with methotrexate if you’re able to properly monitor. 06:35 Do not forget about basal cell carcinoma as well with 5-FU, topical. 06:40 Myelosuppression, not reversible with leucovorin. 06:44 And here, the overdose rescue would be with thymidine, because here, the enzyme that you’re inhibiting is thymidylate synthase. 06:56 6-mercaptopurine, allow the name to speak to you. 06:59 This is a purine drug, a purine analog. 07:02 It decreases de novo purine synthesis. 07:05 It’s activated by HGPRT. 07:07 When’s the last time you’ve heard of HGPRTase, hypoxanthine-guanine phosphoribosyltransferase? Oh yeah, Lesch–Nyhan. 07:15 Right, of course. 07:16 But you need the enzyme to actually activate it. 07:18 It’s a purine analog. 07:20 Clinical use, you should be thinking about something like leukemias and lymphoma, but usually, not CLL or Hodgkin. 07:28 Toxicity here is going to be, well, bone marrow GI. 07:32 And unfortunately, and the reason I say that is if you have a patient that has chronic gout and you give this patient allopurinol, it inhibits xanthine oxidase. 07:45 You require a xanthine oxidase with proper metabolized 6-MP. 07:50 But if you inhibit xanthine oxidase, then oh my goodness, you increase the toxicity of 6-MP. 07:55 Never give 6-MP with allopurinol. 07:59 Thioguanine, what about this one? Same as 6-MP, acute lymphoid leukemia or lymphoblastic leukemia. 08:08 Bone marrow suppression can, however, be given with allopurinol because it’s not metabolized by xanthine oxidase. 08:16 Cytarabine, also known as Ara-C. 08:20 It’s a pyrimidine antagonist. 08:22 Antagonist. 08:24 How does this behave? It inhibits DNA polymerase. 08:27 Make sure you memorize DNA polymerase inhibited. 08:31 AML, ALL, high-grade non-Hodgkin’s lymphoma. 08:36 Here, it may cause leukopenia, megaloblastic anemia, thrombocytopenia, and when you’re dealing with cytarabine, Ara-C. 08:45 In this picture, the graphic illustration of two of the major drugs that we are just referring to. 08:50 5-fluorouracil at the very top, and at the bottom right, you see MTX, which stands for methotrexate. 08:56 5-FU, of course, stands for 5-fluorouracil. 09:00 We said earlier in the table that 5-fluorouracil inhibits thymidylate synthase. 09:04 And in the process, we’ll inhibit the conversion of dUMP into dTMP. 09:09 Remember that uracil is not part of RNA, it has to be thiamine, which is going to be your DNA. 09:15 In the meantime, I want you to go down to the bottom, and we have THF which stands for tetrahydrofolate. 09:21 There’s a methyl form of this, tetrahydrofolate, but more importantly, you’re going to then donate your tetra or two of the hydro -- two dUMP to form dTMP. 09:32 And on the process, you form DHF, which stands for dihydrofolate. 09:37 The recycling of the dihydrofolate requires a reduction chemically by the enzyme, dihydrofolate reductase, which is the enzyme that’s inhibited by methotrexate. 09:47 You understand all this, you’re in good shape. 09:49 I think that you are. 09:50 We saw this earlier as well with megaloblastic anemia. 09:53 And with DHF, remember, that inhibitor, methotrexate, if there’s high dose and you’re using it for, let’s say, that you have whatever type of cancer such as leukemias, then you might want to perhaps rescue by giving this patient leucovorin folinic acid.
About the Lecture
The lecture Cancer Drugs & Chemotherapeutic Agents: MTX, 5-FU, 6-MP, 6TG & Ara-C – White Blood Cell Pathology by Carlo Raj, MD is from the course Leukemia – White Blood Cell Pathology (WBC).
Included Quiz Questions
What is the mechanism of antimetabolites?
- Inhibit DNA replication
- Free radical formation
- Activates dihydrofolate reductase
- Triggers apoptosis
- Alkylates DNA
Which of the given drugs inhibits post-translational modification?
- Etoposide
- Taxols
- Antimetabolites
- Tamoxifen
- Busulfan
Which of the following is inhibited by Etoposide?
- Topoisomerase II
- Thioredoxin reductase
- Thiamine oxidase
- Thymidylate synthase
- Glutathione reductase
Which of the following inhibits dihydrofolate reductase?
- Methotrexate
- Busulfan
- Bleomycin
- Doxorubicin
- Tamoxifen
Which of the following are inhibited by 5-fluorouracil?
- Thymidylate synthase
- Thioredoxin reductase
- Thiamine oxidase
- Topoisomerase II
- Glutathione reductase
What is the mechanism of action of 6-mercaptopurine?
- Decrease in purine synthesis
- Inhibition of topoisomerase II
- Inhibition of DNA polymerase
- Free radical generation
- Inhibition of reductase
What is the mechanism of action of Cytarabine?
- Inhibits DNA polymerase
- Intercalate in DNA
- Inhibits reductase
- Inhibits topoisomerase II
- Free radical formation
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Simple and easy to understand. Dr. Raj explains it well & gives pointers for remembering.