Playlist
Show Playlist
Hide Playlist
Types of IV Fluids (Intravenous Fluids): IV Fluid: 'd5w'
-
Slides TypesofIVFluids RenalPathology.pdf
-
Reference List Pathology.pdf
-
Download Lecture Overview
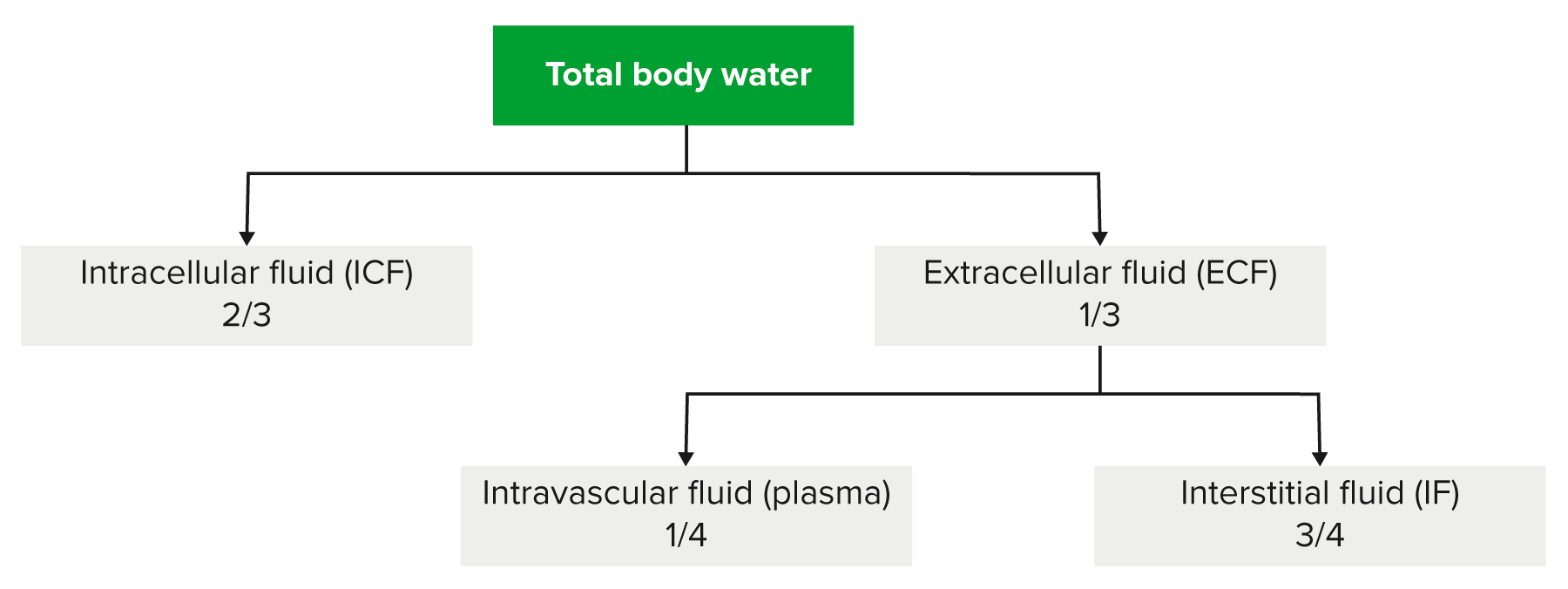
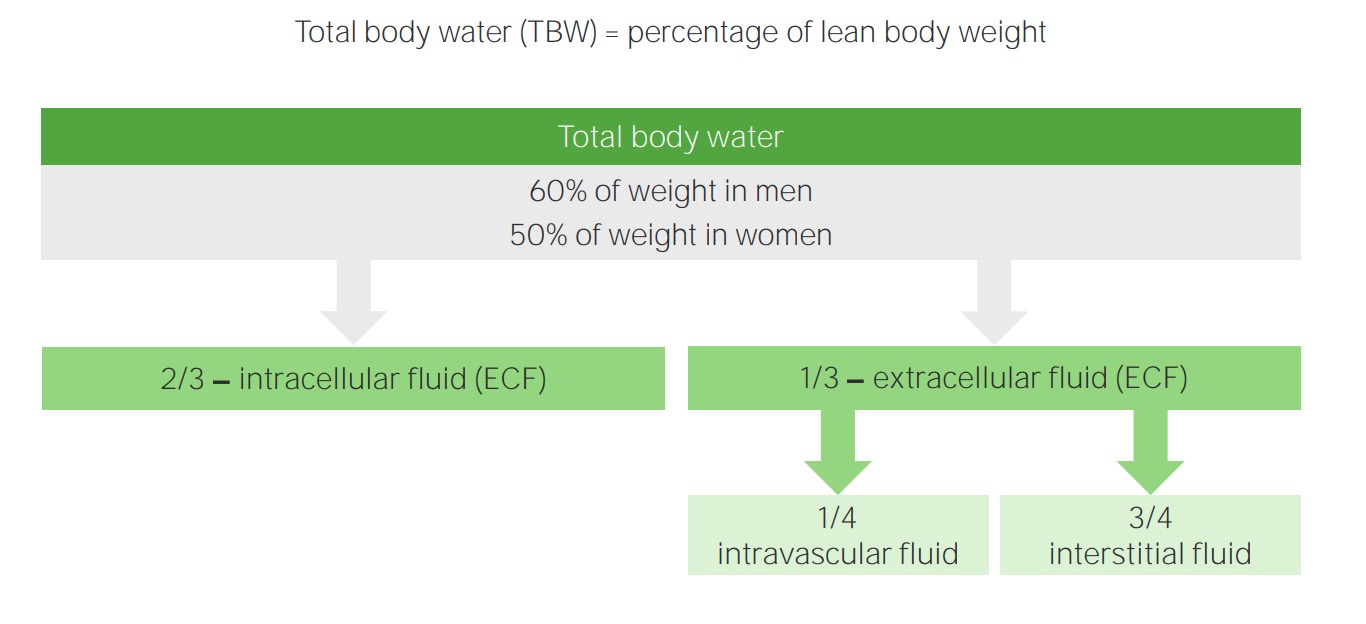
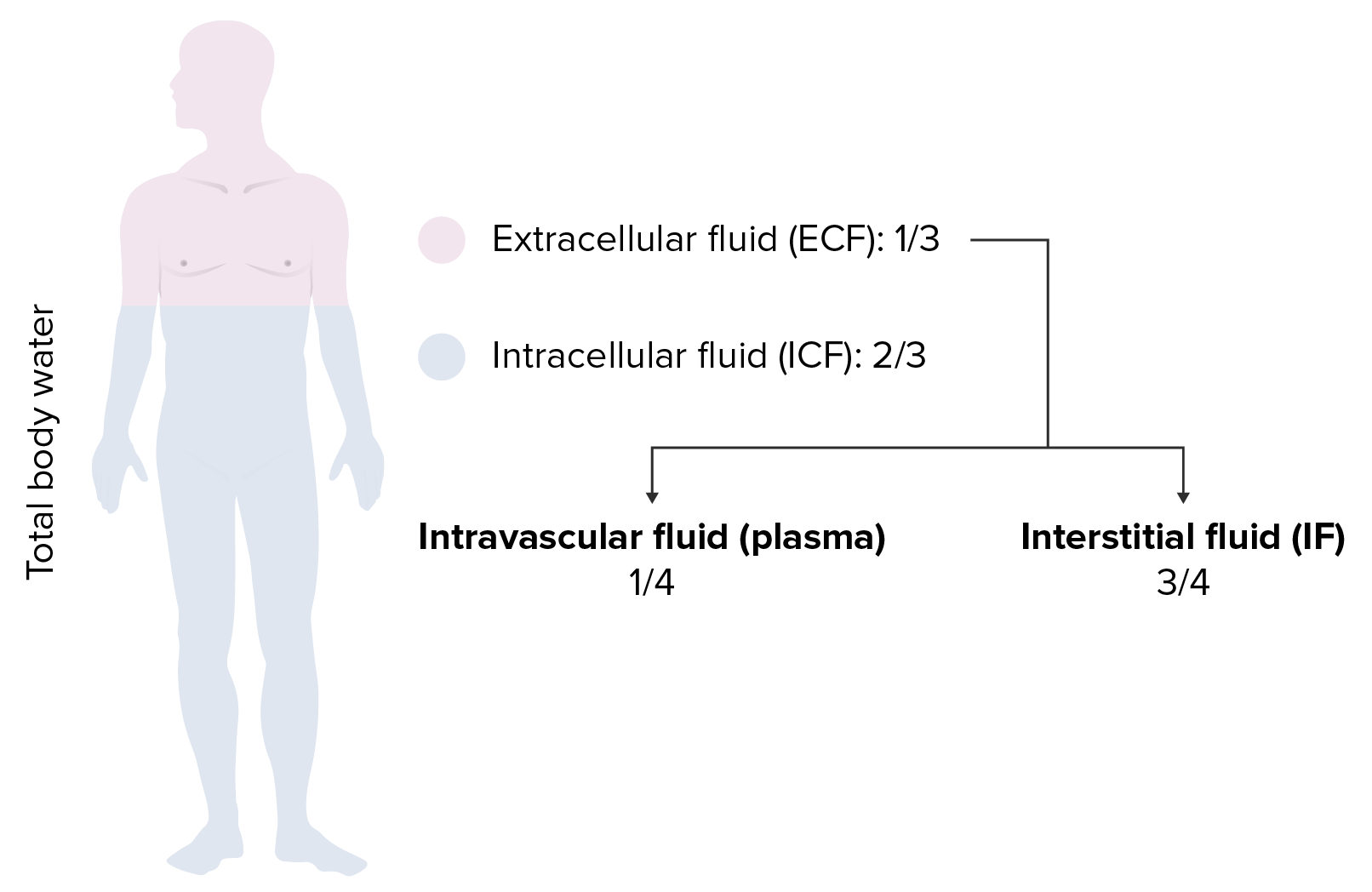
00:00 Here is the D5W. What does this mean to you? Would you ever want to give your patient pure water? Just like that. Would you take a syringe, fill it up with water and here you go, my friend. No, you are not Kevorkian, your are not turned to euthanasia, that is not your job on your boards and wards right now. Your job is to keep the patient alive please, on your clock. So you are not going to give your patient pure water because if you do so, where you are putting that water straight into? Right into the cell. The cell is going to swell and it will die. Okay. So you don't want to do that. But there might be situations where you might want to give D5W and the reason that you give D5, which is 5 percent dextrose is because it will slow down the water's entry into the cell, meaning to say that dextrose will get metabolized in the ECF and water will remain obligated. 00:57 Once that dextrose gets dissolved, then the water gets into the cell, but not like the rush it would have been meaning like a water fall or drinking out of fire hydrant killing you. So this would be manageable. So let's do D5W. It's isotonic to serum, we have glucose dextrose rapidly metabolized leaving the pure water. Why do you want this? So that you don't kill the cell very quickly. It is equivalent to giving one litre of pure water and the statement here is telling you exactly as to what you want to avoid. Pure water cannot be administered because if you did then it will enter the cell immediately causing ICF shift and causing cell swelling and lysis. Pure water will distribute evenly within total body water. What does that mean to you? Tell me the fractions really quick. 01:49 2/3, 1/3, where we are paying attention to the 1/3 are referring to the ECF equilibrate between those two compartments. For example, let us say, we have 1 liter of D5W. Now once again put on your thinking cap. We don’t use math, we don't use math on a consistent basis. But when we do use it at that point you do want to be alert. Just to make sure you cross your T's and dot your I's. We have 1 litre pure water that you are giving. We are going to equilibrate between ECF and ICF, what are you going to pay attention to? The ECF. So here we have 333 of ECF. Of this, how much is then going to enter your plasma? 83, 1/4, bottom line okay. Big difference between D5W and normal saline. Normal saline, 1 liter well that distributes between your plasma and your interstitium 3/4, 1/4. And if you gave 1,000, how much of it ended up in the intravascular space? What is 1/4 of your 1,000? If it was normal saline. One litre. 03:03 Yeah 250. Here you got them easily 83. This is as difficult as to some of this math will get for you, necessary though, so that you understand your IV fluids. What is normal saline? Crystalloid or is it a colloid? Good. 03:20 So you have your NaCl which is your crystalloid.
About the Lecture
The lecture Types of IV Fluids (Intravenous Fluids): IV Fluid: 'd5w' by Carlo Raj, MD is from the course Fluid and Electrolyte Balance.
Included Quiz Questions
Which of the following statements about D5W is INCORRECT?
- It remains within the extracellular fluid compartment.
- It is hypotonic in the body.
- It is distributed across total body water.
- Glucose is rapidly metabolized leaving pure water.
- It is equivalent to giving a patient pure water.
What is the reason pure water is never infused into a patient?
- Osmotic gradient would result in cell swelling and lysis
- It distributes solely into the intracellular fluid compartment.
- None of the answers are correct
- Pure water would result in cell shrinkage.
- Pure water is able to cross all membranes.
How much fluid would remain in the plasma compartment from an infusion of 1 L of D5W?
- 83 mL
- 250 mL
- 167 mL
- 667 mL
- 333 mL
How much fluid would remain in the extracellular compartment after an infusion of 1 L of D5W?
- 333 mL
- 750 mL
- 83 mL
- 200 mL
- 500 mL
Which of the following statements about D5W is CORRECT?
- Distributes evenly within total body water
- It is hypertonic in the body.
- It distributes within the intracellular compartment.
- Dextrose is not rapidly metabolized.
- It is not equivalent to giving a patient pure water.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |