Playlist
Show Playlist
Hide Playlist
Thromboregulation
-
Slides Thromboregulation Thrombolysis Normal Hemostasis.pdf
-
Reference List Pathology.pdf
-
Download Lecture Overview
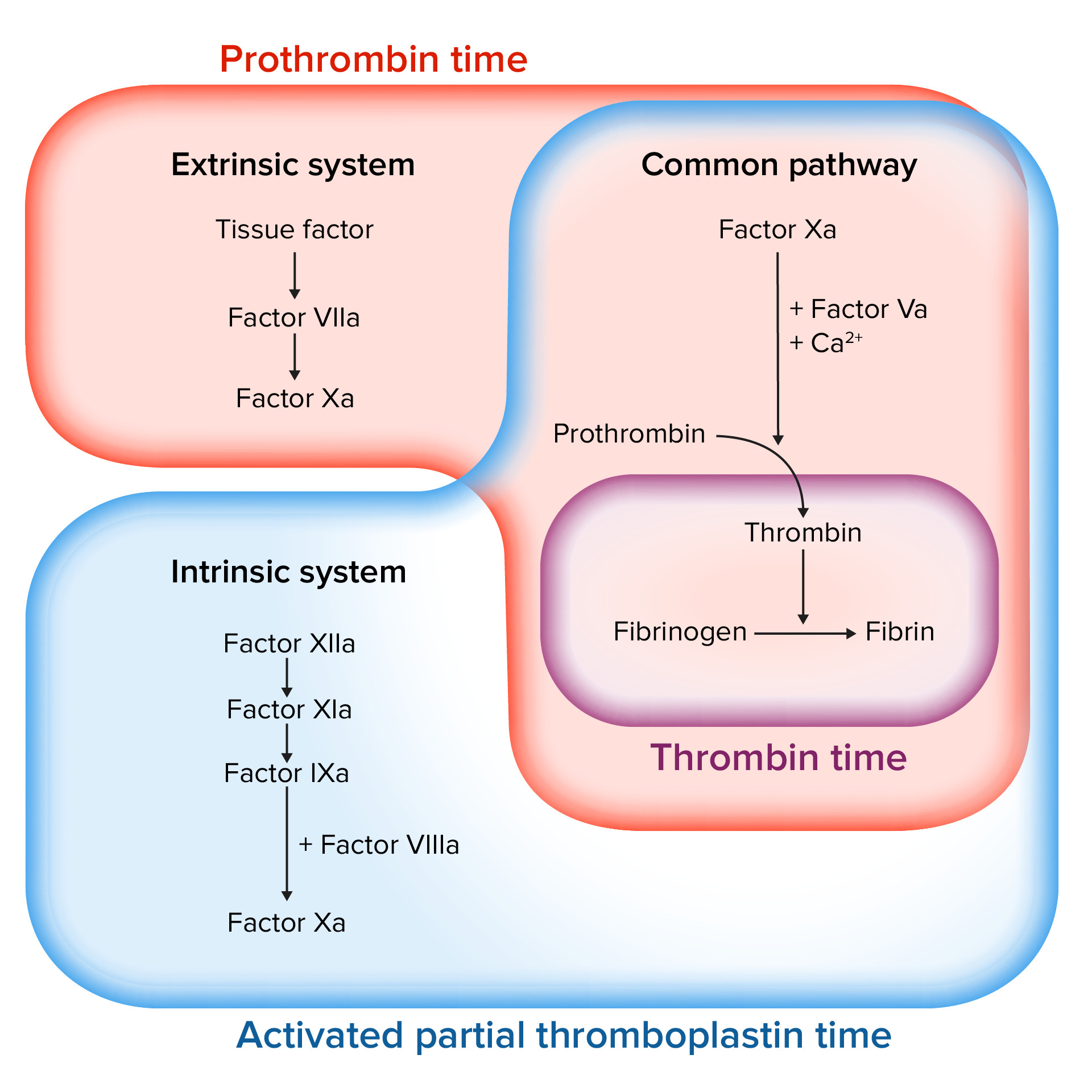
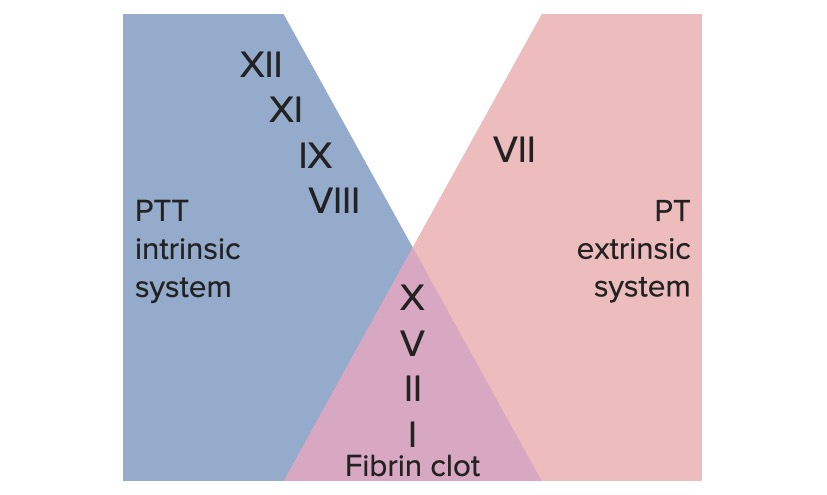
00:00 Alright, so we have talked about normal hemostasis. Let's get down into some more weeds and talk about how we control thrombus breakdown. Here's the road map that we've been working off. We talked about normal hemostasis, we talked about normal thrombus formation. Even as we were talking about normal thrombus formation, we are also already talking about the feedback pathways that limit the formation of thrombus, but we're going to talk a little bit more in detail about that now. So we want to limit the thrombus to the injured site and we want to begin to break down the clot. That's the fibrinolysis. So we're going to cleave the fibrin that's cementing the platelets together so that we can eventually restore completely normal flow to the vessel once everything is healed. How do we limit things to just the site where we want them? Remember, all those coagulation factors synthesized by the liver are flowing through all parts of the bloodstream and if we activate them kind of willy-nilly and then they wander off some place else, they could potentially deactivate coagulation factors somewhere that's completely inappropriate where there is no injury. So how do we limit the coagulation activation to just our site of injury? So we're going to look at just one of the examples about this. This is working through the extrinsic pathway. So remember tissue factor, factor VII getting into the common pathway and we can talk about the conversion of factor II to factor IIa. This, as we had noted previously when we talked about the coagulation factors, is happening on a surface that has phospholipid. They are blue balls with the long tails and is happening with calcium so that we can everything all binding in one area. So what we have is factor VIIa, that's inactivated coagulation factor from the extrinsic pathway we have tissue factor. Sitting in association with this phospholipid membrane provided by platelets in most cases and everything is being held together by calcium. Along comes now are circulating factor X. It's inactivated. But because tissue factor in VIIa are limited to where they can be because of their necessity to bind to the phospholipid and calcium, we can only get factor X activation to Xa at that site. So we can limit that, we can make sure it only happens where we wanted. 02:46 And now, we can bring in cofactor Va, again requiring phospholipid, again requiring calcium, the little yellow dots, and now that activated factor Xa can act on factor II that would otherwise be just flowing by in the circulation and we're binding it all up into one location so we can activate it just at that site. So it's a very clever way that we can make sure that we only activate the coagulation cascade. Even though it's circulating factors wheezing by, it will only happen at the sites of injury where we have already aggregated the platelets and provided the polyphosphates and the membrane of the platelet to provide that substrate. So that's very clever. We can also limit the size of the thrombus by making other factors and we've already talked about a heparin-like molecule synthesized by endothelial cells that interacts with a circulating antithrombin III or ATIII. That activity will limit now the proteolytic activities of XII and XI and IX and X. So we can limit the size of the thrombus with other factors, and antithrombin III is a very important one. Actually it's important therapeutically as we'll talk about in a couple of sessions because heparin that we administer to all of our patients if they're going to be hospitalized and inactive for a long period of time, we give them heparin. That's so that we can keep antithrombin III maximally active to limit clot formation just because patients are lying around and inactive. So, very important therapeutically that we keep that in mind. Remember too that when thrombin gets activated it then will act to the endothelial cells and will induce the formation of thrombomodulin. Thrombomodulin will convert thrombin to something that will now become an anticoagulation factor and it will cleave protein C making activated protein C interacting with cofactor protein S and now we're going to turn off VIIIa and Va. So this is another way that we can limit, even with circulating factors, we can limit the activation of the coagulation cascade. And the final one really very important because it has therapeutic implications is a molecule called plasmin. And plasmin, as we'll see on the next slide, is responsible for cleaving fibrin. So even though we have made fibrin fibrils and we have cemented the platelets until we crosslink them, we can still break them down and that's the job of plasmin.
About the Lecture
The lecture Thromboregulation by Richard Mitchell, MD, PhD is from the course Hemostasis.
Included Quiz Questions
What substances play an essential role in the extrinsic pathway?
- Calcium ions and factor VIIa
- Calcium ions and factor IXa
- Calcium ions and factor IV
- Calcium ions and factor II
- Calcium ions and factor VIII
Antithrombin III activity is enhanced by...
- ...heparin.
- ...plasmin.
- ...factor XII.
- ...prothrombin.
- ...factor XI.
What cleaves the fibrin clot?
- Plasmin
- Antithrombin III
- Heparin
- Activated protein C
- Tissue factor
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |