Playlist
Show Playlist
Hide Playlist
Tertiary Structure – Peptides
-
03 Basic Peptides.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
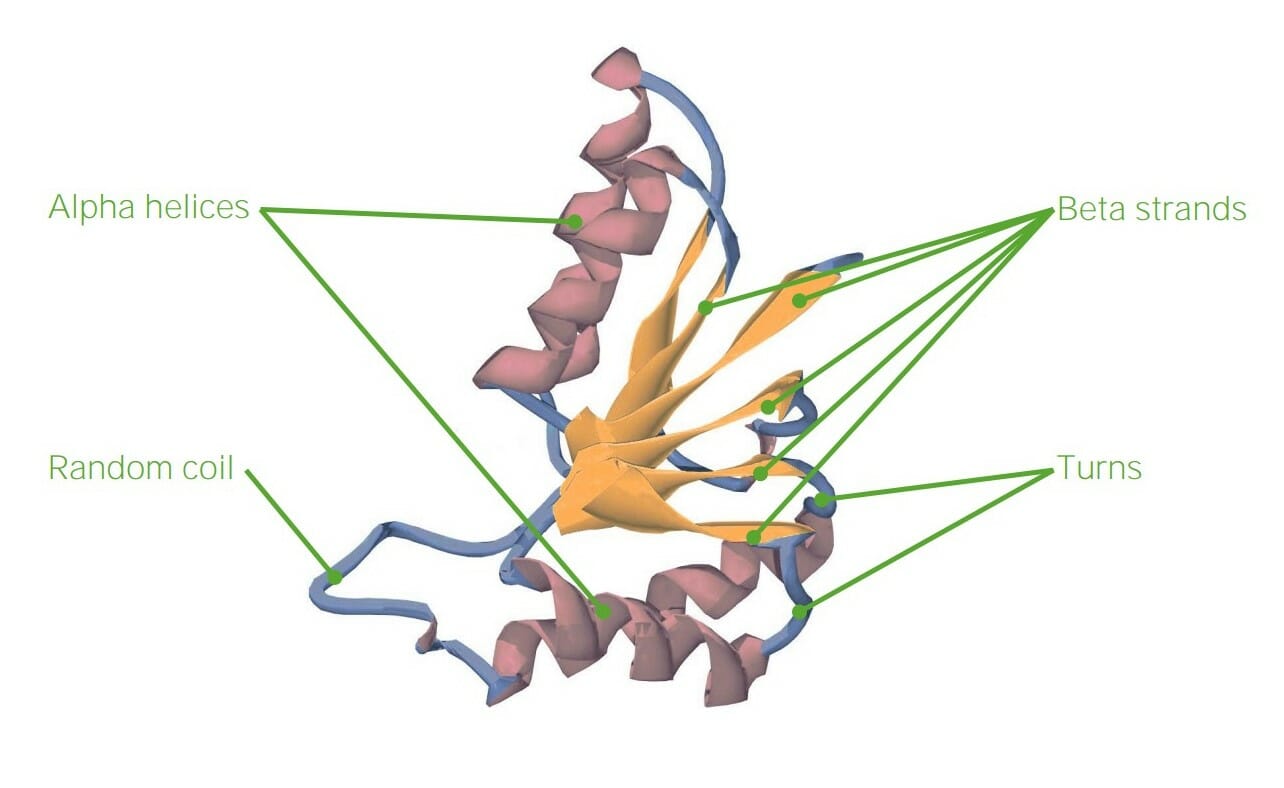
00:00 some of the proteins and their fibrous nature as you can see on the screen here. 00:00 Now when we get to tertiary structure, predicting tertiary structure from primary sequence is almost impossible to do, at least with computing and our understanding of this process today. 00:12 We see this protein that has alpha helices and we also see within the protein, the yellow regions called beta strands that have been organized to form a sheet. The reverse turns that I described earlier are shown at this point and there's yet another feature of this protein that's very important, it's called the random coil. The random coil is a part of the protein that doesn't have a specific structure. It's not random in the sense that it doesn't go off on tangents, but what it does, is it doesn't form a regular repeating structure like I've already described. Because of random coils and because of variabilites within the alpha helices, beta strands and reverse turns, predicting tertiary structure proteins is very, very difficult to do. 01:03 Now tertiary structure proteins arise as a result of folding, meaning that we can have a linear sequence of amino acids that ultimately come together and make what we describe as a globular protein. Now globular proteins are called that, I like to tell students, because if you were to look at them in a macroscopic world, you would look at that and say, that's a glob of something, because they're all folded up and while that folding appears to be quite random, it is in fact quite specific. The sequence of amino acids in the primary structure will determine ultimately the folding that a protein does. Now the folding of a protein gives the protein its characteristic functions, whether it's catalysis, whether it's structure, or whether it's other functions that it's performing. In primary structure, we said that the peptide bond was the stabilizing force that held together the amino acids. 01:58 In secondary structure we said that hydrogen bonds held together alpha helices and also the beta strands. When we get to tertiary structure, we discover that there are different forces, in addition to hydrogen bonds, that help to stabilize tertiary structure and they are shown in this schematic figure that shows a protein that has folded, albeit in an unusual way. We start on the right side of this figure, showing an alpha helical region of the protein and we're reminded that hydrogen bonds stabilize alpha helices. The next region of the protein is also stabilized by hydrogen bonds, but these hydrogen bonds are occurring between amino acids that are not close to each other in primary sequence. And so when we have interactions that arise between sequences that are not close in primary sequence, that means they must be tertiary by definition. 02:54 The disulfide bonds become a factor in tertiary structure. Disulfide bonds, you may remember, are bonds that arise as a result of interactions between the sulfhydryls of two cysteine groups. 03:07 When these two cysteine groups get into close proximity, they will form a covalent bond called the disulfide bond that you see in this structure. Now disulfide bonds are covalent bonds and therefore, are the strongest bonds that help to stabilize protein structure. 03:26 Moving further to the left and at the bottom, we can see interactions arising between positively charged amines and negatively charged carboxyl groups. These are R groups of the individual proteins, either let's say a basic amino acid like lysine with the amine group, and a R group carboxyl, like aspartic acid that they have ionized and are interacting as a result of charge attractions to each other. These ionic bonds can play a very important role in organizing and helping to stabilize a tertiary structure. Now another bond that occurs in tertiary structure, that is a little bit harder to understand, is that of a hydrophobic bond. The hydrophobic bond is shown above the ionic bonds and you can see the side chains of isoleucine, valine and phenylalanine that are all interacting with each other. Now what does this mean? Each of these amino acids is fairly hydrophobic, meaning it has a side chain that does not like to interact with water. Globular proteins are commonly soluble in the cytoplasm of the cell, meaning abundant water. Well since these amino acids have side chains that don't like to interact with water, they will tend to avoid water and they will tend to interact with each other, instead of interacting with water. Very much like oil, when you mix it with water, will separate and associate with itself and not associate with the water. Now it's interesting that when we compare different proteins and we look at the location of the hydrophobic amino acids, for proteins that are soluble in water, proteins will fold, so as to prefer the location of the hydrophobic amino acid side chains on the interior part of the protein. And that's because again, in avoiding water, they get stability, and that stability translates to stability for the protein. The last of the individual bonds that help to stabilize proteins that I'll discuss here are those of metallic bonds and in this particular figure you can see an iron atom that has stabilized two regions of a protein.
About the Lecture
The lecture Tertiary Structure – Peptides by Kevin Ahern, PhD is from the course Biochemistry: Basics.
Included Quiz Questions
Which of the following correctly describes a disulfide bond?
- Covalent bond
- Ionic bond
- Hydrogen bond
- Hydrophobic bond
- Metallic bond
Which is the strongest bond in a tertiary structure?
- Disulfide bond
- Hydrogen bond
- Hydrophobic bond
- Ionic bond
- Metallic bond
The disulfide bond occurs between which of the following?
- Two cysteine amino acids
- Two methionine amino acids
- Methionine and cysteine amino acids
- Methionine and proline amino acids
- Cysteine and aspartic acid amino acids
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |