Playlist
Show Playlist
Hide Playlist
Structure of Proteins – Protein Movement and Cell Signaling
-
04 Basic ProteinMovement&CellSignaling.pdf
-
Biochemistry Free and Easy.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
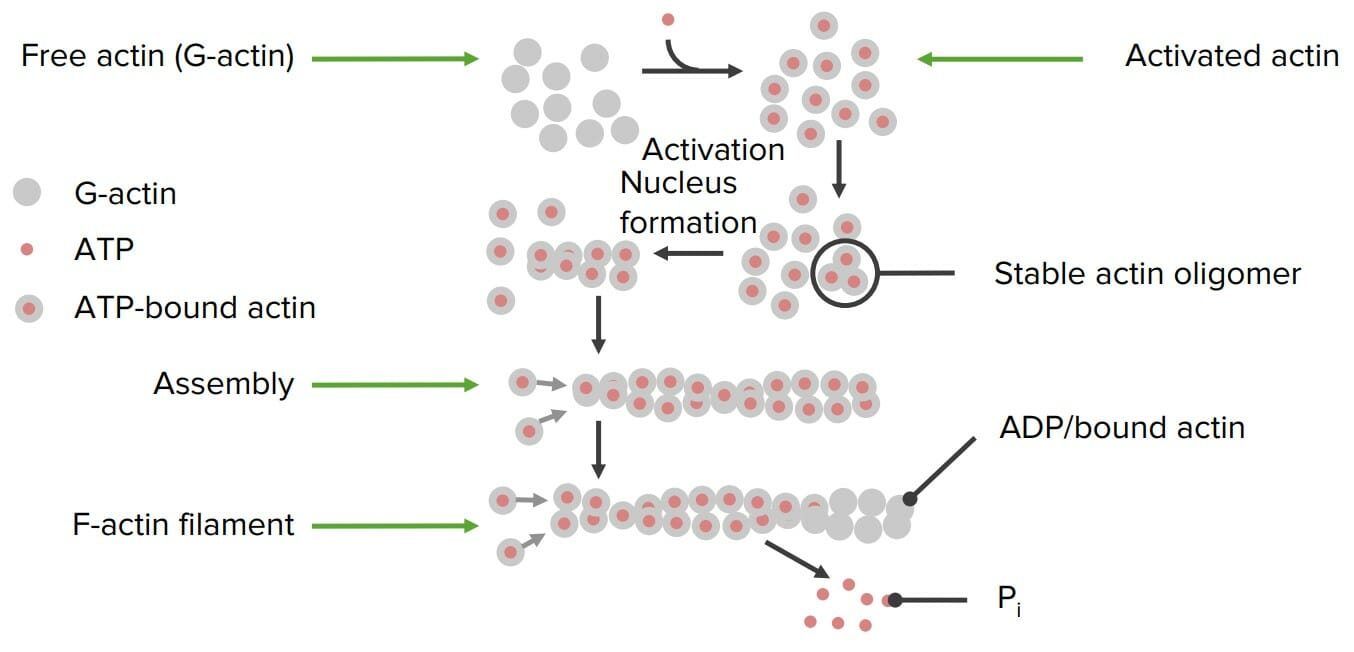
00:01 Mit Ausnahme der Codierung der genetischen Information sind Proteine an praktisch jeder wichtigen Funktion der Zelle beteiligt oder führen diese aus. In dieser Vorlesung werde ich über die Funktionen von Proteinen in Bezug auf Struktur, Kommunikation, Signalübertragung und Bewegung sprechen. Bei Proteinen gibt es sehr wichtige Überlegungen, die wir in Bezug auf ihre Gesamtstruktur anstellen. 00:25 Proteine sind, wie wir wissen, sehr wichtige Polymere aus Aminosäuren, und eine der interessantesten Eigenschaften von ihnen ist ihre Flexibilität. Dank ihrer Flexibilität sind Proteine in der Lage, Dinge wie Katalyse, erstaunlich effizient, und andere Prozesse durchzuführen, die es ihnen ermöglichen, sich anzupassen, wie wir noch sehen werden. 00:42 Die Struktur von Proteinen ist wichtig, insbesondere die Struktur der einzelnen Aminosäuren, aus denen Proteine bestehen. Proteine können zum größten Teil entweder polar oder unpolar und geladen sein. Diese verschiedenen Polaritäten oder Ladungen bestimmen, wo ein Protein in einer Zelle angeordnet ist. 01:07 Proteine können eine Vielzahl von Formen haben. Sie können faserig sein, was bedeutet, dass sie eine sich ziemlich regelmäßig wiederholende faserartige Struktur haben. Sie können kugelförmig sein, was auf eine Faltung hinweist, durch die ein Protein seine endgültige Struktur erhält, und schließlich können Proteine entweder als Fasern oder als kugelförmige Proteine in Membranen eingebunden sein. 01:29 Jetzt werde ich hier über die faserigen Proteine sprechen. Faserproteine sind sehr wichtig für die Dinge in unserem Körper. Die Keratinproteine zum Beispiel sind Proteine, aus denen unsere Haare und unsere Nägel bestehen. Diese Faserproteine, über die ich sprechen werde, zeichnen sich dadurch aus, dass sie sehr robust und langlebig sind, und wir wissen, dass unsere Nägel und die Krallen von Vögeln zum Beispiel sehr, sehr zähe Materialien sind. Kollagen ist ein faseriges Protein, das im Wesentlichen der Klebstoff ist, der uns zusammenhält. Es bildet den Knorpel und das Bindegewebe. Fibroin ist das Protein, aus dem Seide besteht, und auch es ist sehr widerstandsfähig. Andere Faserproteine sind Elastin oder Fibrillin, die an unserem Bindegewebe beteiligt sind, und Lamin, das für die Struktur der Kernhülle verantwortlich ist. Die anderen Proteine, aus denen die Fasern bestehen, sind nicht das, was wir als Faserproteine bezeichnen. Es handelt sich vielmehr um kugelförmige Proteine, die polymerisieren, um Fasern zu bilden. Dazu gehört Aktin, ein kugelförmiges Protein, das an der Bildung des Zytoskeletts beteiligt ist. Ein weiteres ist Tubulin, das kleine Internet-Autobahnen in unseren Zellen bildet, auf denen sich Proteine bewegen können. Dies sind ebenfalls kugelförmige Proteine, und diese durchlaufen ebenfalls einen Selbstfindungsprozess. 02:50 Nun, faserige Proteine, die keine inhärente faserige Natur eingebaut haben, sind Proteine die überwiegend eine Sekundärstruktur, aber praktisch keine Tertiärstruktur haben. Dazu gehören die Keratine, aber auch andere Proteine wie z.B. Kollagen. Wenn wir die Sequenz der vorhandenen Aminosäuren analysieren, stellen wir fest, dass es sich weitgehend um Sequenzwiederholungen handelt. 03:13 Wenn wir uns zum Beispiel die Fibrin-Wiederholungsstruktur oben rechts ansehen, können wir feststellen, dass die Wiederholungsstruktur Glycin, Serin, Glycin, Alanin, Glycin, Alanin und viel Glycin enthält. Ähnlich verhält es sich mit der nachstehenden Teilsequenz des Kollagens, die ebenfalls reichlich Glycin und darüber hinaus reichlich Prolin und eine modifizierte Aminosäure namens Hydroxyprolin enthält. Glycin ist also reichlich vorhanden, und das wahrscheinlich aus einem sehr guten Grund, denn Glycin ist flexibel. Das Hydroxyprolin im Kollagen ist bemerkenswert, weil es sich um eine modifizierte Aminosäure handelt, d. h. die Aminosäure Prolin wird zunächst in das Protein eingebaut, aber später wird an das Prolin eine Hydroxylgruppe angehängt. Dies scheint ein relativ unbedeutender Faktor für die Gesamtstruktur eines Proteins zu sein, aber bei Kollagen erweist er sich als sehr wichtig. Die Hydroxylierung der Prolinreste im Inneren des Kollagens hilft ihnen, sich miteinander zu verbinden, und wenn sie sich miteinander verbinden, verleiht dies dem resultierenden Kollagen eine große Festigkeit. 04:17 Deshalb kann unser Kollagen die Dinge zusammenhalten. 04:24 Interessanterweise wird für die Reaktion, bei der die Hydroxygruppe an Prolin gebunden wird, Vitamin C benötigt. 04:30 Es ist seit vielen Jahren bekannt, dass ein Mangel an Vitamin C zu einer Krankheit führt, die als Skorbut bekannt ist, und Skorbut tritt im Wesentlichen wegen des Kollagens auf. Menschen fallen buchstäblich auseinander, wenn sie einen Mangel an Vitamin C haben.
About the Lecture
The lecture Structure of Proteins – Protein Movement and Cell Signaling by Kevin Ahern, PhD is from the course Biochemistry: Basics.
Included Quiz Questions
Which of the following is true of fibrous proteins?
- They commonly contain abundant amounts of glycine.
- They derive most of their properties from their catalytic nature.
- They have an extensive tertiary structure.
- They are composed of almost all random sequences.
- They are very fragile.
Which of the following fibrous proteins is NOT correctly associated with its location in the body?
- Elastin — cell membrane
- Keratin — hair
- Collagen — cartilage
- Lamin — nuclear envelope
- Fibrillin — connective tissue
What is the significance of hydroxyproline?
- The stabilization of the structure of collagen
- The transportation of collagen from the nucleus to the cytoplasm
- The assembly of ribosomes on the mRNA
- The transportation of collagen from ER to Golgi bodies
- The catalytic reaction carried out by the collagen in the lysosomes
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Excellent information and explanation, it's helping me a lot. Congrats, lecturio!