Playlist
Show Playlist
Hide Playlist
Shape and Isomerism – Biological Interactions
-
Slides 10 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
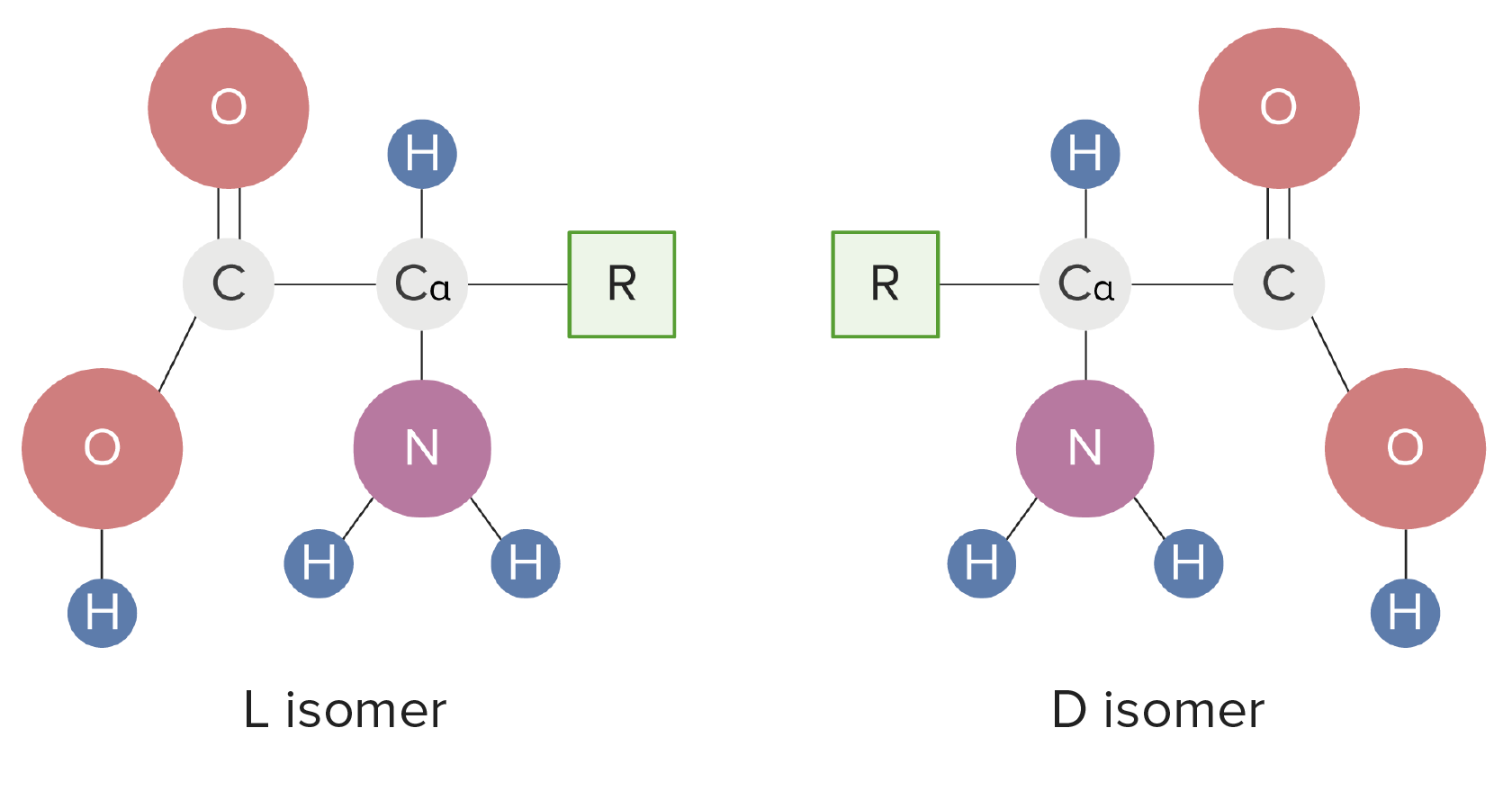
00:01 Let’s have a quick look at shape and isomerism. 00:05 In order for drugs to fit an active site, they must first have the correct shape. 00:09 And we made reference to this earlier on. 00:12 Remember what I said about, for example, the planar nature of aromatic rings. 00:16 Sometimes a planar shape is absolutely essential to correctly bind to a lipophilic pocket within a given receptor or enzyme. 00:25 Usually, only one isomer of a drug will have the required activity. 00:29 And by that, I don’t just necessarily mean optical isomers. 00:34 Most natural products, however, do have stereocentres and occur in a single optical isomer, otherwise known as an enantiomer, enantiomer. 00:45 For example, we have the amino-acid which make up proteins and hence, receptors and enzymes. 00:53 However, drugs should be administered as single enantiomers as the mirror images may have other effects including producing side effects, which are unwanted, countering the effects of the drug, which is something that you actually see, for example, in the antidepressant, citalopram, where it is administered as a racemate. 01:13 One enantiomer of the drug, the S or sinister enantiomer, has been shown to have a pronounced effect in the treatment of depression. 01:21 However, the R enantiomer has actually been shown to counteract the effect of the S. 01:28 Also, there is always a risk that one of the drugs or one of the enantiomers can be metabolised to a toxic product because the enzymes involved in metabolism are also quite specific about the types of things they break down and the way in which certain drugs are converted into metabolites. 01:48 So, let’s look at thalidomide, just briefly, as an example. 01:53 Developed in the 1950's as a sedative and was found and was used as an over-the-counter to treat nausea in pregnancy. 02:01 However, it was found that the drug caused devastating abnormalities to the children of mothers taking thalidomide, the most common being the limb malformation. 02:12 So, looking at the structures, you can see that they are, to all intents and purposes, well, identical. 02:17 They contain the same number of carbons, the same number of electrons, the same number of hydrogens, oxygens and nitrogens. 02:24 However, investigation at the time showed that it was the R, or rectus, enantiomer that causes the sedation whereas the S, or sinister, isomer causes birth defects. 02:37 So, that on the right, was responsible for the limb malformation that was observed. 02:42 In the case of thalidomide, formulation of the R isomer alone, however, was not sufficient to remove the danger. 02:51 And this is because the drug can racemise in the body. 02:55 And this is facilitated by a biological process in which epimerisation of that stereogenic centre occurs. 03:03 And you get conversion of the R to the S. 03:08 So, unfortunately, even in this particular scenario, administering one particular enantiomer would not have overcome the effects that were observed, in terms of the negative effects, as a teratogen. 03:22 Thalidomide, however, has made a reappearance as it’s now being used against leprosy and as an anti-cancer drug. 03:30 So, bearing in mind, it was prescribed for pregnant mothers and therefore, this would have been an issue, but for other members of society who would be suffering, for example, from leprosy or certain types of cancer, thalidomide still has a role to play. 03:47 Now, I just want to briefly touch upon geometrical isomerism. 03:51 We were made aware of this earlier on when we were talking about alkenes in Module III when we looked at the idea of cis- and trans-. 04:01 And this type of isomerism, just to recap, is where you, being unable to rotate around a double bond, get two groups which are the same on either one side, such as in the case of maleic acid shown on the left, which is cis- and fumaric acid, which is trans, where two groups are on either of that double bond. 04:23 There is no free rotation around a double bond under normal conditions. 04:28 It is, however, possible to induce the rotation by applying light of a certain frequency which, if you’re really interested in finding out more, I recommend you look at the photo-isomerism of stilbene. 04:43 The functional groups in this case essentially end up in different places and you’ll see it referred to either as cis- or zusammen or z and trans- or entgegen or e. 04:56 And, finally, conformational isomerism. 04:59 So, remember what I said before about the idea of fitting particular molecules because of their shape into a particular cleft. 05:06 And this is cyclohexane. 05:08 You can see its idealised, regular hexagon structure, but the reality is it exists either as the boat or as the chair. 05:17 So the chair structure, which is the preferred structure, maximises the distance between hydrogen atoms and carbon atoms, therefore, reducing the degree of steric interaction between different electron clouds within those molecular orbitals. 05:31 The problem when you’re thinking about this though is, if you have a narrow cleft which only a planar molecule can fit, because this is actually a kinked hexagon and not through a regular hexagon, this would render this interaction of higher energy rather than a lower energy interaction, which is what you want to achieve. 05:52 If the drug has a preferred confirmation which fits the active site then it will usually bind more easily than if it is needed to adopt an alternative shape.
About the Lecture
The lecture Shape and Isomerism – Biological Interactions by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
Why is a particular conformation of drug molecules preferred in pharmaceutical sciences?
- Because the conformation of the drug molecule affects the efficient binding between the drug molecule with its receptor.
- Because the conformation of the drug molecule facilitates the attack of water molecule on the electron dense areas of the receptor molecule.
- Because the conformation of the drug molecule facilitates the recognition of receptor molecule for the attack of drug metabolizing enzymes.
- Because the conformation of the drug molecule allows the racemization of the drug molecule to another conformation type.
- Because the conformation of the drug molecule facilitates the breakdown of the drug molecule after it binds to a metabolizing enzyme.
Drugs are usually administered in single enantiomeric form. Why?
- To minimize side-effects related to the other enantiomeric form of the drug.
- To minimize enzymatic metabolism of the drug molecules.
- To help the drug escape the cell-mediated immune response of the host body.
- To prevent digestive enzymes from attacking sensitive groups on the another enantiomeric form of the drug.
- To help the drug escape the antibody-mediated immune response of the host.
Which of the following is NOT true regarding thalidomide?
- The use of thalidomide is highly recommended in pregnant women to prevent limb malformations.
- Thalidomide can have teratogenic effects.
- The human body enzymes convert S-enantiomer of thalidomide into the R-form via epimerization reaction.
- Thalidomide is a promising drug for leprosy and malignant tissues.
- The R-enantiomer of thalidomide serves as a sedative, whereas S-enantiomer is teratogenic.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |