Playlist
Show Playlist
Hide Playlist
Serine Family and Methionine Catabolism
-
Slides AminoAcidMetabolism Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
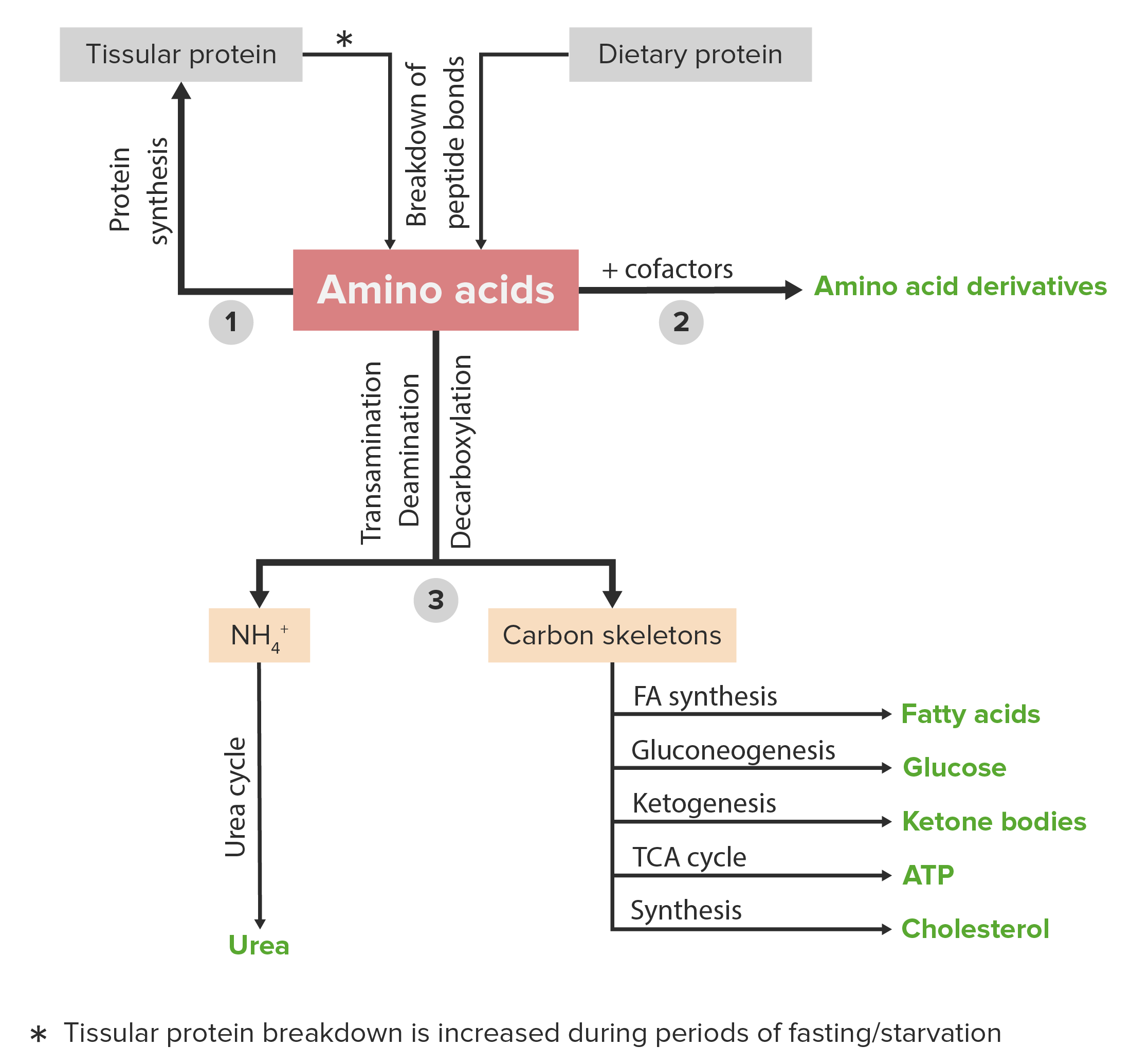
00:01 The next amino acid metabolism family I want to consider is that of the serine family. 00:07 So, the serine family, as the other families are described, uses serine as a central amino acid for branching out to make the other amino acids. 00:17 So to understand this family, I need to first describe how serine is synthesized. 00:21 There were two main pathways that lead to serine in our cells. 00:25 The first one starts form 3-phophoglycerate. 00:28 Now, this molecule, if you recall, is found in the glycolysis pathway so we see a linkage between glycolysis and this amino acid metabolism. 00:37 The reaction starts with an oxidation of the 3-phophoglycerate as we can see here. 00:42 In this reaction, 3-phosphohydroxypyruvate is produced as a result of that oxidation. 00:48 The transamination of 3-phosphohydroxypyruvate leads to orthophosphoserine or O-phosphoserine as we can see here. 00:58 And the removal of phosphate from O-phosphoserine results in the production of the amino acid serine. 01:04 It's a very simple set of steps that make that. 01:08 A second way of making serine starts with glycine. 01:12 And this is a set of reactions that I've described in other lectures here relating to folate metabolism. 01:18 This is a very important reaction not only for making serine but also in the reverse direction for making glycine, as well as interchanging the formation of different folates as we will see. 01:29 So, here's the reaction that occurs. 01:32 We start in this case with glycine and we start with this rather complicated folate name N5, N10-methylene tetrahydrofolate, mouthful. 01:41 This reaction, a CH2OH group from the methylene tetrahydrofolate is transferred onto glycine to make serine. 01:51 The product of that transfer is tetrahydrofolate. 01:54 So remember from folate metabolism that this is a way of interchanging the different folates. 01:59 The reaction as I said can go on the reverse direction and the reverse direction serine is used as a precursor to make glycine. 02:07 The CH2OH group that is added to serine is shown in the green box. 02:13 The next amino acid whose metabolism we want to consider in the serine family is that of cysteine. 02:18 Cysteine we remember is one of two amino acids that contains a sulfur. 02:22 So getting this sulfur into this serine backbone is a central part of making cysteine. 02:28 Now, cysteine can be made in multiple ways. 02:31 And when we see amino acids made in multiple ways, it suggests first of all an interconnection with a lot of other metabolic processes, but it also illustrates the importance of that amino acid. 02:41 Cysteine is a very important amino acid for making proteins. 02:45 The primary means of making a cysteine is tied to the metabolism of methionine. 02:51 So I'll start with that process. 02:53 If we look at methionine, methionine of course is the other amino acid that contains a sulfur. 02:59 But methionine in this process is actually donating a methyl group as we can see. 03:04 Methionine is used to make S-adenosylmethionine at a rather complicated reaction that shown here. 03:11 The adenylyl part of ATP combines with methionine to make S-adenosyl methionine or otherwise known as SAM. 03:19 The enzyme catalyzing this reaction is methionine adenosyltransferase as you can see here. 03:24 In the second step of the process, S-adenosylmethionine is converted to S-adenosylhomocysteine or SAH. 03:31 Now this reaction involves the donation of a methyl group to something else, right? It's not going to make cystein but it's making an intermediate that will be used to make cysteine. 03:41 That intermediate is S-adenosylhomocysteine. 03:44 The enzyme catalyzing this reaction is transmethylase. 03:48 In the next step, hydrolyzing S-adenosylhomocysteine to release the adenosine creates the molecule homocysteine. 03:55 And I've drawn its structure on the right. 03:58 The enzyme catalyzing this is S-adenosylmethionine hydrolase. 04:03 Now, homocysteine is an important molecule to understand because it has numerous health consequences. 04:10 High blood levels of homocysteine is related to cardiovascular disease and stroke risk. 04:16 And so one of the things physicians will do when they're assessing your health is actually measure the level of this molecule, because high levels of this molecule are not good. 04:25 In the next step, homocysteine combines with serine to make cystathionine. 04:30 Now, I've drawn the structure of this molecule on the lower right. 04:33 And we'll in just a second how cysteine is made from that. 04:38 The enzyme catalyzing this reaction is cystathionine beta-synthase. 04:42 Now, deficiency of this enzyme leads to homocystinuria. 04:46 And we've seen that homocysteine is not a good molecule to have. 04:49 So a deficiency of this enzyme has some pretty severe consequences for a person's health. 04:55 And the last reaction, cystathionine is converted into cysteine Now, this reaction involves splitting out of alpha-ketobutyrate, and the splitting out of that results in the production of cysteine. 05:09 Now, this is slightly complicated so I'll show you that in just a second. 05:13 Water is required for this process and the process, not only is the splitting occurring that produces cysteine, but the other half of the molecule is losing an ammonium group to become the beta-ketobutyrate. 05:25 The enzyme catalyzing this reaction is cystathionase. 05:28 And we can see what has really happened in this process. 05:32 So I've started here to show you the starting molecules that make cystathionine. 05:37 I've labeled, first of all, the serine and the homocysteine. 05:43 Now, these two combine together to make the cystathionine What happens in producing cysteine is simply we shift where we cut. 05:51 You can see that in this case that the boxes of the green and the red are shown in the right side of the sulfur to cleave out and make cysteine involve simply shifting the box. 06:02 So the cleavage happens as shown here and that's what produces cysteine, as shown in the green, and beta-ketobutyrate is produced in the molecule on the right after water cleaves off the ammonium ion.
About the Lecture
The lecture Serine Family and Methionine Catabolism by Kevin Ahern, PhD is from the course Amino Acid Metabolism. It contains the following chapters:
- Serine Family
- Methionine Catabolism
Included Quiz Questions
Which of the following glycolysis intermediates is the closest precursor to serine?
- 3-phosphoglycerate
- Dihydroxyacetone phosphate (DHAP)
- 2-phosphoglycerate
- Pyruvate
- Phosphoenolpyruvate (PEP)
Which of the following amino acids is serine most closely linked through folate metabolism?
- Glycine
- Alanine
- Cysteine
- Arginine
- None of the above
The deficiency of which enzyme leads to homocystinuria?
- Cystathionine β-synthase
- Methionine adenosyltransferase
- S-adenosyl methionine hydrolase
- Transmethylase
- Cystathionase
Cystathionine is converted into cysteine and β-ketobutyrate by which enzyme?
- Cystathionase
- Methionine adenosyltransferase
- S-adenosyl methionine hydrolase
- Transmethylase
- Cystathionine β-synthase
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |