Playlist
Show Playlist
Hide Playlist
Respiratory Acidosis & Alkalosis
-
Slides 09 ToolsCompensation AcidBaseBalance GeneralPhysiology.pdf
-
Download Lecture Overview
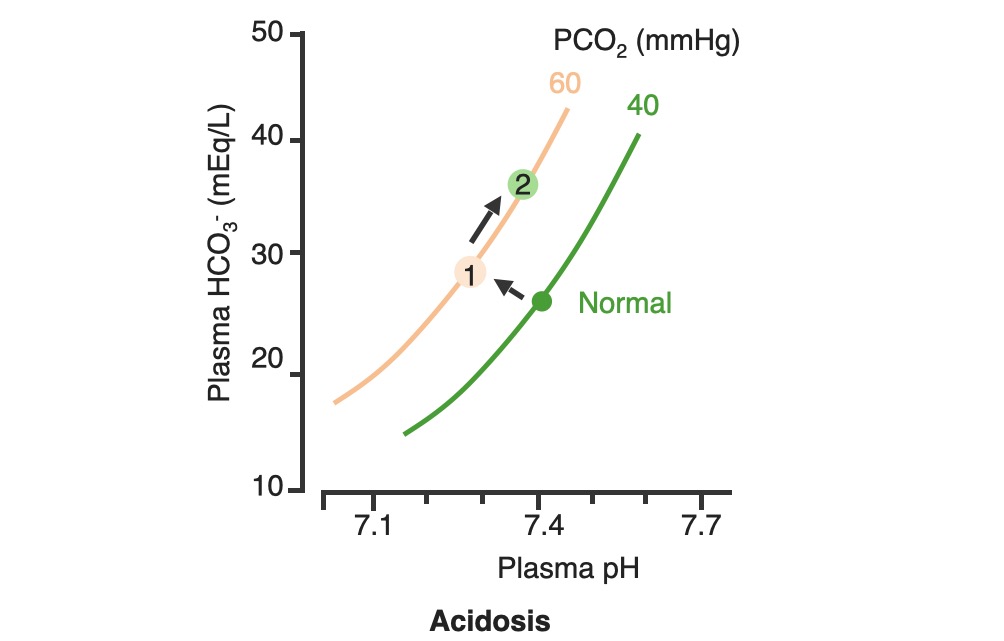
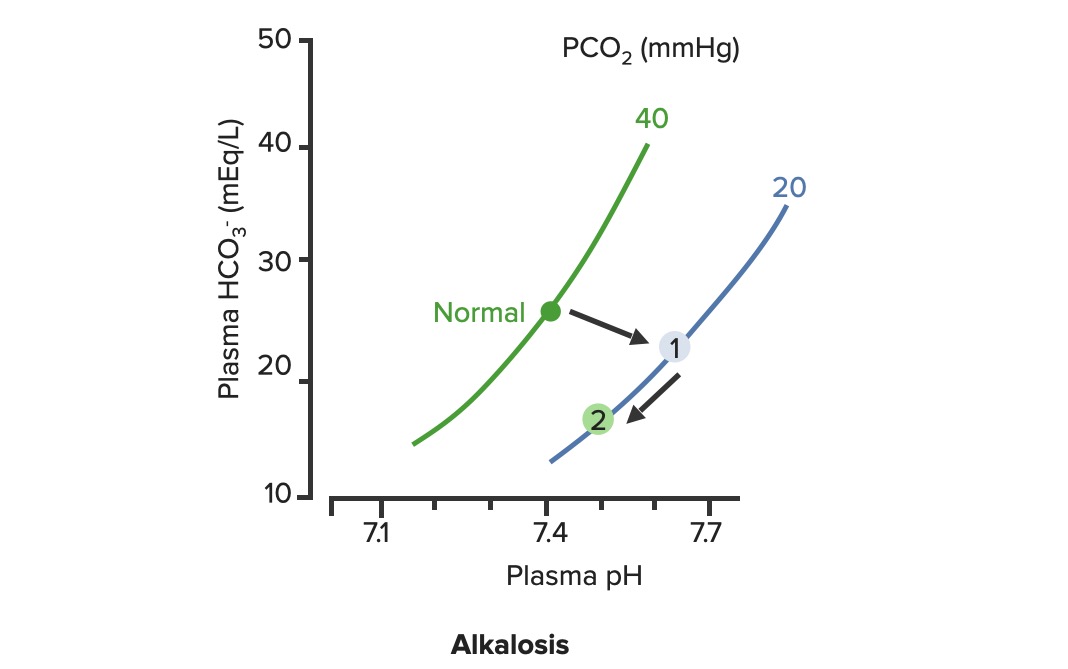
00:01 Great! Now, that we have the acid base box down. 00:04 Let’s talk through some disorders that could cause an acidosis from the respiratory side of things. 00:11 If you have a decrease of alveolar ventilation that will cause a respiratory acidosis. 00:16 There are some examples of these such as if you have a neuromuscular disorder such as ALS. 00:22 If you have, Chronic Obstructive Pulmonary Disease could also cause a decrease in alveolar ventilation. 00:31 You can also have decrease in the diffusion capacity of the lung such as interstitial fibrosis. 00:37 would cause this particular response. 00:40 Another way you can have a respiratory acidosis if you have a severe ventilation-perfusion mismatch. 00:48 Severe ventilation-perfusion mismatches is sometimes occur in things like chronic bronchitis, or very severe asthma, or even acute edema. 00:58 So this are all potential ways you would get respiratory acidosis. 01:04 But on something like an acid-base box what will we be concerned about for this respiratory problem? PACO2 So if you have a respiratory acidosis you’ll be building up carbon dioxide. 01:19 So you would have a high PACO2. 01:23 and that is what you are looking for, for respiratory acidosis. 01:28 So why do I worried about ventilation when I am thinking about CO2 values? Well, alveolar ventilation (VA) and PACO2 are inversely related. 01:40 What do I mean by inversely related? If one goes up, the others gonna go down. 01:44 If one goes down, the other ones gonna go up. 01:47 So lets take a look at this. 01:49 If you have a hypoventilation, you have a decrease in alveolar ventilation, automatically you have an increase in PACO2. 01:58 The opposite also occurs. 01:59 If you have, a hyperventilation, you have an increase in alveolar ventilation, automatically you have a decrease in PACO2. 02:08 Now, it doesn’t matter if this is alveolar CO2 or arterial. 02:13 So we could use PACO2 or PaCO2, either would work fine. 02:19 There is an inverse relationship. 02:22 Why is this so handy? Because if you measure someone’s PACO2 with an arterial blood gas, you automatically know what there ventilation status is. 02:34 If you measure the low PACO2, you know that their alveolar ventilation rate was high. 02:40 If you measure a high PaCO2, you always know that there alveolar ventilation rate was too low. 02:49 Respiratory acidosis and alkalosis always works in this manner. 02:56 You notice that haven’t really gone through a lot of respiratory alkalosis problems, right? We went through three respiratory acidosis, right. 03:05 A decrease in AVO ventilation, a problem with the diffusion capacity, and a severe ventilation of perfusion mismatched. All respiratory acidosis issues. 03:16 Respiratory alkalosis, the only thing that happens is you breathe too much. You hyperventilate. 03:23 That is why you would have a respiratory alkalosis. 03:27 So you could force yourself to do that by deep breathing. 03:32 Those large breathes and deep breathes you could breathe out a lot of your CO2 cos you increase alveolar ventilation, PCO2 will drop. 03:43 Okay, so lets now use a different tool rather than in the acid-base box. 03:48 We’ll use an acid base diagram. 03:52 Now this has a lot of information on it and its really nice this allows you to predict what might happen in response to a change in something like ventilation rate. 04:03 So we have three different axis to deal with. 04:06 We have Plasma pH, we have Plasma bicarb and then along this isopleths, which is just a fancy word for this, curvilinear line that goes up. 04:17 PCO2 and this is PaCO2 or the partial pressure carbon dioxide in the arterial blood. 04:26 Normally, you have a PACO2 of 40 mmHg. 04:31 You have a plasma pH of 7.4, a bicarb of 24. 04:37 So that’s your normal. 04:39 The normal is now seeing here as a green dot. 04:42 So the normal condition, PCO2 of 40, Plasma pH of 7.4, bicarb level of 24. 04:50 So that is where our starting point is. 04:53 If we are going to increase our PaCO2, that is an increase in carbon dioxide. 05:01 That happens if you don’t ventilate enough. 05:05 So that is a decrease in alveolar ventilation. 05:07 We also call that hypercapnia. 05:10 So in these case, you are travelling from the normal line which is the green dot up to the hypercapnia line which is now at 60 mmHg. 05:23 We travel up via a linear line. 05:27 That linear line is known as blood-buffer line. 05:30 That blood-buffer line was previously define for us as 25 millimolar per unit of pH (25 mM /pH unit). 05:39 This is the buffering ability of whole blood. 05:42 So things like hemoglobin and other plasma proteins always allow you to travel not linearly but having a small slope associated with it. 05:53 The other thing I will point out is that bicarb levels rose slightly but not a lot. Just a little bit. 06:03 That little increase in bicarb is simply the product of the carbonic anhydrase equation. 06:10 And I’ll explain it a little bit more after we go through to the hypocapnic condition. 06:16 So now, let’s increase alveolar ventilation. 06:19 So now, we are going to go and breathe off more CO2. 06:24 In these case, we are gonna move now to these blue line still following the blood-buffer line. 06:30 You’d increase alveolar ventilation which blows off more CO2 and causes hypocapnia. 06:37 You notice in this condition you have an increase in plasma pH. 06:43 That’s because if you look at the dot, the blue dot, drop the vertical line down, you would see that pH increased from 7.4 to very close to 7.6. 06:55 Now, lets deal with this bicarb issue again. 06:59 Because when they said the blood-buffer line doesn’t have bicarbonate, you still might get changes in bicarb. 07:06 it’s a very small change but there is a small decrease in bicarbonate levels. 07:12 And that is a hard thing to kind of wrap over your head around. 07:16 Because anytime that you have an increase in hydrogen ions, you will always have a small increase in bicarb. 07:26 Anytime you have a decrease in hydrogen ions you will always have a small decrease in bicarb. 07:32 Because you’re following the same equation. 07:35 What I mean by the same equation? You take carbon dioxide plus bicarb water. 07:43 You get carbonic acid and then that product yields a hydrogen ion and a bicarb. 07:50 So however this formulate its traveling in one direction, if you get a change in hydrogen ions you’re always gonna get a change associate with its product. 08:01 Now a large change, what a change? That is why you give a small change in each of those variables as you go from hypo to hypercapnia. 08:12 In terms of how the body responds to a change in alveolar ventilation. 08:18 So these is the hyper and hypocapnia that we’ve talk about. 08:21 How does the body deal with these? You usually deal with the problem by using another system. 08:28 So if it’s a respiratory problem you try to compensate with the renal system. 08:34 So lets go through an example for that. 08:37 If you have a hypercapnic condition, meaning you haven't a decrease an alveolar ventilation. 08:44 You’are gonna go from the normal line up to point number one. 08:49 However, to then try to compensate for that, you are going to now travel up the PC02 line. 08:57 As you travel up the PCO2 line, this is important, you are not travelling along the blood-buffer line anymore. 09:05 you are traveling up the PCO2 line. 09:09 These dramatically increases bicarb. 09:14 not just a little bit but a dramatic increase. 09:17 If you take point number two and drop a vertical line down, you will notice that pH is now returning towards normal. 09:25 So if you started off with the pH of 7.4, maybe moved to a pH of maybe 72.5. 09:35 Now, you’re moving back closer to 7.4 again. 09:41 The same thing can happen in a time in which you have a low PCO2. 09:49 So if you now move from normal to 0.1, that is moving down the blood-buffer line. 09:55 How does the body try to compensate for that? You dump bicarb. 09:59 And you do that at the level of the kidney and you go from 1 to 2. 10:05 That is a response a renal compensation to a respiratory problem. 10:14 If we use the acid-base box, we can do this also with acidotic conditions. 10:22 So here, we have an arterial blood gas of 7.34. 10:27 Red gnome, outside the box. 10:29 BICARB below 22? Red gnome outside the box. 10:36 What do we have left? Blue gnome outside the box on the opposite side. 10:42 By knowing where the gnomes are, I can name this condition It is metabolic acidosis with respiratory compensation. 10:51 How do you know that? You always name the condition base upon where the gnomes are. 10:58 If there are 2 gnomes on one side, that’s how you name it. 11:03 Metabolic acidosis with respiratory compensation. 11:08 Lets take another example. 11:10 Lets do another acidosis. 11:13 So pH, I mean ABG 7.24. 11:18 Red gnome, outside the box. 11:20 So you know it’s an acidosis. 11:22 Right now, you know it’s an acidosis. 11:25 Now, were you doing is waiting your waiting, you are at the edge of your seat. 11:29 Your thinking, okay is it gonna metabolic? is it gonna be respiratory? How would you know? The gnome would be outside the box on the same side is the red headed gnome. 11:38 So let’s look where we at? the bicarb levels are of 23. 11:45 You have a gnome inside the box. 11:48 Here you are not sure what to do. 11:49 Green gnome inside the box. It’s gonna be hard to name. 11:52 Red gnome outside the box respiratory weather This is respiratory acidosis With no metabolic compensation.
About the Lecture
The lecture Respiratory Acidosis & Alkalosis by Thad Wilson, PhD is from the course Acid-Base Balance.
Included Quiz Questions
If renal compensation occurs in response to a dramatic increase in alveolar ventilation, what change is predicted with bicarbonate?
- A dramatic decrease in HCO₃₋
- A small decrease in HCO₃₋
- A small increase in HCO₃₋
- A dramatic increase in HCO₃₋
Which of the following does not decrease alveolar ventilation?
- Pulmonary embolism
- Chronic obstructive pulmonary disease
- Neuromuscular deformities
- Chest wall deformity
- Obesity
Which of the following would be very high in a patient with respiratory acidosis?
- PaCO₂
- pH
- HCO₃
- PO₂
- Respiratory dead space
What is the blood buffer line defined as?
- 25 mmol/unit of pH
- 35 mmol/unit of pH
- 15 mmol/unit of pH
- 20 mmol/unit of pH
- 30 mmol/unit of pH
Which of the following compounds is the most effective buffer system at a physiological pH of 7.4?
- Bicarbonate buffer
- Protein buffer
- Ammonia buffer
- Phosphate buffer
- All of the above
Which one of the following conditions will not cause respiratory alkalosis?
- Laryngeal obstruction
- Fever
- Anxiety
- Complication of type 1 diabetes mellitus
- Salicylate toxicity
A patient has atelectasis and is experiencing respiratory acidosis. Which of the following would be the expected blood gas values in this patient?
- pH 7.25, PaCO₂ 50 mmHg
- pH 7.35, PaCO₂ 40 mmHg
- pH 7.50, PaCO₂ 52 mmHg
- pH 7.52, PaCO₂ 28 mmHg
- pH 7.56, PaCO₂26 mmHg
A child has a panic attack and breathes rapidly. Which of the following should we expect?
- Respiratory alkalosis
- Metabolic acidosis
- Respiratory acidosis
- Metabolic alkalosis
- Normal acid-base balance
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
3 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Thank you! I appreciate how you explain this material. Thank you
Dr. Wilson does an excellent job in breaking down concepts of acid-base balance in the body and making it plain. He also provides some additional tricks/tools that you may not get in your regular lectures, but will definitely help on an exam. These lectures are top notch, just excellent!
1 customer review without text
1 user review without text